April 2014 | VOL. 13, NO. 4 | www.McGowan.pitt.edu
Platelet-Rich Plasma and Fat Grafting: An Option?
A Leader in Immunogenetics
As reported by McGowan Institute for Regenerative Medicine faculty member co-authors J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, UPMC Endowed Professor of Plastic Surgery, and Professor of Bioengineering at the University of Pittsburgh, Co-Director of the Adipose Stem Cell Center, and is Founder and Director of the Center for Innovation in Restorative Medicine in the Department of Surgery at the University of Pittsburgh, and Kacey Marra, PhD, Associate Professor, Department of Surgery at the University of Pittsburgh, Director of the Plastic Surgery Laboratory, and Co-Director of the Adipose Stem Cell Center, autologous fat grafting is an important treatment option for small to medium soft tissue defects derived from tumor ablation, congenital deformity, and traumatic injury. The advantages are that autologous fat is easy to obtain in large quantities and the procedure is less uncomfortable and risky to patients; the operation is of short duration, can sometimes be performed under local anesthesia, and simultaneously achieves an aesthetic result in both the donor and recipient sites. However, the disadvantages of fat grafting are an unpredictable and variable reabsorption rate of around 40%–60%, resulting in the need for repeated procedures, and microcalcifications and cyst formation due to fat necrosis. Reabsorption and fat necrosis are believed to be caused by insufficient neoangiogenesis around the fat graft, thus resulting in adipocyte apoptosis due to lack of nutrient supply and accumulation of metabolic waste.
Several strategies have been reported to enhance fat graft survival, such as adjunct therapy by adding the stromal vascular fraction (SVF), enhancing angiogenesis by addition of growth factors, or use of chemical cell-stimulating factors, such as insulin or erythropoietin. Among these, platelet-rich plasma (PRP) has recently emerged as a new matrix to enhance fat graft survival.
PRP, which is derived from whole blood through double-spin centrifugation, contains multiple growth factors and adhesion molecules in α-granules. PRP is believed to be safer and more practical in clinical adjunctive therapy than other recombinant growth factors or stem cell therapies. In addition, PRP is an economic way to obtain multiple growth factors at one time that meet the requirements for highly complex processes during tissue repair or regeneration. PRP has been demonstrated to be effective in bone regeneration, wound healing, and improvement of musculoskeletal sports injuries.
Recently, clinicians extended the scope of PRP therapy to soft tissue augmentation by combining PRP with fat grafting. Although some successful clinical results were reported, evidence supporting the application of PRP combined with fat grafts in soft tissue augmentation remains limited, as only a few basic research and preclinical studies in small animals have been conducted. Furthermore, no details on molecular mechanisms are addressed in the literature.
In their review article in Tissue Engineering–Part B, Review, Drs. Rubin and Marra discuss the possible molecular mechanisms of PRP in fat graft survival based on a review of the current literature. This review discusses published in vitro, animal, and human studies and provides guidance for future research and clinical application.
RESOURCES AT THE MCGOWAN INSTITUTE
May Special at the Histology Lab
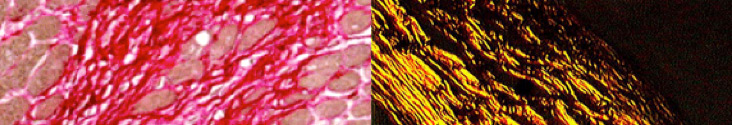
Break out the tanning oils and sunglasses or have summer fun the histology way: with immersion oil and polarized lenses.
Picro-Sirius Red is a Connective Tissue Stain intended for use in the histological visualization of collagen I and III fibers in addition to muscle in tissue sections. The PSR stain may be viewed using standard light microscopy or polarized light resulting in birefringence of the collagen fibers to distinguish between type I and type III.
You’ll receive 30% off your PSR stain every day in May. Any day in May is a good Day for PSR Staining when you mention “summer fun” while dropping your order off at the McGowan Institute Histology Core Facility.
Contact Lori at the McGowan Core Histology Lab and ask about the Summer Fun in Histology.
Email perezl@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology in the lowest amount of time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
Study Challenges Accepted Sepsis Treatment
 A structured, standardized approach to diagnose and treat sepsis in its early stages did not change survival chances for people who develop this deadly condition, according to a national, randomized clinical trial led by experts at the University of Pittsburgh School of Medicine including McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Distinguished Professor and Mitchell P. Fink Chair, Department of Critical Care Medicine at Pitt.
A structured, standardized approach to diagnose and treat sepsis in its early stages did not change survival chances for people who develop this deadly condition, according to a national, randomized clinical trial led by experts at the University of Pittsburgh School of Medicine including McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Distinguished Professor and Mitchell P. Fink Chair, Department of Critical Care Medicine at Pitt.
Their findings, available in the New England Journal of Medicine, could change the way sepsis is diagnosed and treated. Each year, sepsis, the body’s response to severe infections, kills more people than breast cancer, prostate cancer, and HIV/AIDS combined.
“We found no overall differences in two protocolized approaches when compared to conventional treatment. The study provides h3 evidence that will have immediate consequences,” said Dr. Angus, investigator of the study. “Many organizations have endorsed structured guidelines for sepsis treatment that often call for invasive devices early in care. But with prompt recognition and treatment of the condition, we found that these approaches do not improve outcomes but do increase the use of hospital resources,” said Dr. Angus.
The 5-year, multicenter study, called “Protocolized Care for Early Septic Shock” or ProCESS, was sponsored by an $8.4 million grant from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health (NIH).
“More than 750,000 cases of severe sepsis and septic shock occur in the U.S. each year, most receiving care initially in an emergency department. We’ve found that if early recognition and treatment happen, one approach to supporting circulation while giving antibiotics is not better than another,” said Donald M. Yealy, MD, a ProCESS investigator and Professor and Chair of Pitt’s Department of Emergency Medicine.
“ProCESS set out to determine whether a specific protocol would increase the survival rates of people with septic shock. Instead, it showed something far more important—that over the past decade, the care of people with sepsis has significantly improved nationwide,” said Sarah Dunsmore, PhD, who managed the ProCESS trial for NIGMS. “ProCESS showed that regardless of how the delivery of the interventions was monitored, sepsis patients in these clinical settings are receiving effective treatments.”
Between March 2008 and May 2013, 1,351 patients with septic shock at 31 U.S. hospital emergency departments were enrolled in the trial. They were randomly chosen to receive EGDT, PSC, or usual care for the first 6 hours of resuscitation.
The researchers found no difference in outcomes among the three interventions: at 60 days post-intervention, 21 percent of the EGDT group, 18.2 percent of the PSC group, and 18.9 percent of the standard care group had died in the hospital. There also were no differences in mortality after 90 days or 1 year.
“There have been many improvements in the management of sepsis in the past decade. We examined whether giving the medical team step-by-step instructions to monitor and treat the effects of sepsis could improve survival rates as the previous study suggested,” Dr. Angus said. “EGDT, PSC, and usual care all offer early diagnosis and methods to deliver fluids, restore blood pressure, and monitor cardiovascular function; one was not better than the other to treat the condition effectively.” Added Dr. Yealy, “We are not suggesting that sepsis care should be delayed or can be limited.”
Model-Based Decisions in Sepsis (MODS)
 A team of University of Pittsburgh researchers including McGowan Institute for Regenerative Medicine affiliated faculty members recently received funding for an R01 proposal from the National Institute of General Medical Sciences of the National Institutes of Health (NIH). The title of the proposal is “Model-Based Decisions in Sepsis (MODS)” and the amount is $800,000 of direct costs through 2018. The team (in alphabetical order) includes:
A team of University of Pittsburgh researchers including McGowan Institute for Regenerative Medicine affiliated faculty members recently received funding for an R01 proposal from the National Institute of General Medical Sciences of the National Institutes of Health (NIH). The title of the proposal is “Model-Based Decisions in Sepsis (MODS)” and the amount is $800,000 of direct costs through 2018. The team (in alphabetical order) includes:
- Derek Angus, MD, Professor and Chair of the Department of Critical Care Medicine and Director of CRISMA (Clinical Research, Investigation, and Systems Modeling of Acute Illnesses) Center at the University of Pittsburgh (co-principal investigator)
- Gilles Clermont, MD, Associate Professor of Critical Care Medicine and Medical Director of the Center for Inflammation and Regenerative Modeling at the University of Pittsburgh (principal investigator)
- Robert Parker, PhD, Associate Professor, Chemical and Petroleum Engineering, University of Pittsburgh, and Member of the Molecular Therapeutics and Drug Discovery Program, University of Pittsburgh Cancer Institute (co-principal investigator)
- Francis Pike, PhD, Assistant Professor, Critical Care Medicine, University of Pittsburgh School of Medicine, and Department of Biostatistics, University of Pittsburgh (co-principal investigator)
- David Swigon, PhD, Assistant Professor of Mathematics and an Associate Member in the Department of Computational Biology, University of Pittsburgh (co-principal investigator)
The Research Project (R01) grant is an award made to support a discrete, specified, circumscribed project to be performed by the named investigator(s) in an area representing the investigator’s specific interest and competencies, based on the mission of the NIH.
Illustration: Wikipedia.
New “Zero-Dimensional” Carbon Nanotube May Lead to Superthin Electronics and Synthetic Cells
 Synthetic, man-made cells and ultrathin electronics built from a new form of “zero-dimensional” carbon nanotube may be possible through research at the University of Pittsburgh Swanson School of Engineering co-directed by McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, chair of the Department of Chemical and Petroleum Engineering, associate professor, and Bicentennial Alumni Faculty Fellow of the University of Pittsburgh Swanson School of Engineering, and McGowan Institute affiliated faculty member Anna Balazs, PhD, the distinguished Robert Von der Luft professor of chemical and petroleum engineering. The research, ““Zero-Dimensional” Single-Walled Carbon Nanotubes,” was recently published in the journal Angewandte Chemie.
Synthetic, man-made cells and ultrathin electronics built from a new form of “zero-dimensional” carbon nanotube may be possible through research at the University of Pittsburgh Swanson School of Engineering co-directed by McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, chair of the Department of Chemical and Petroleum Engineering, associate professor, and Bicentennial Alumni Faculty Fellow of the University of Pittsburgh Swanson School of Engineering, and McGowan Institute affiliated faculty member Anna Balazs, PhD, the distinguished Robert Von der Luft professor of chemical and petroleum engineering. The research, ““Zero-Dimensional” Single-Walled Carbon Nanotubes,” was recently published in the journal Angewandte Chemie.
Along with co-principal investigators Drs. Little and Balazs, co-investigators include Riccardo Gottardi, PhD, Ri.MED Foundation Fellow, whose research focuses on nanotechnology and biomedical engineering; Alexander Star, PhD, associate professor of chemistry; Bhaskar Godugu, PhD, research assistant professor and director of Pitt’s mass spectrometry facility; Susheng Tan, PhD, research assistant professor; postdoctoral researchers Yanan Chen, PhD and Kaladhar Kamalasanan, PhD; and Sam Rothstein, PhD, CSO and co-founder of Qrono Inc.
“Since its discovery, carbon nanotubes have held the promise to revolutionize the field of electronics, material science, and even medicine,” says Dr. Little. “Zero-dimensional carbon nanotubes present the possibility to build ultrathin, superfast electronic devices, far superior to the best existing ones and it could be possible to build h3 and ultralight cars, bridges, and airplanes.”
One of the most difficult hurdles is processing the carbon nanotubes into smaller forms. However, previous research at Pitt has managed to cut the carbon nanotubes into the smallest dimensions ever to overcome this problem.
The organization of the atoms within nanotubes makes them particularly interesting materials to work with. However, they are barely soluble, making industrial processing difficult. One aspect of the team’s research will focus on creating more soluble and therefore more usable carbon nanotubes. These shorter nanotubes have the same dimensions as many proteins that compose the basic machinery of living cells, presenting the potential for cell- or protein-level biomedical imaging, protein or nucleic acid vaccination carriers, drug delivery vehicles, or even components of synthetic cells.
Overall, the project is aimed at developing and working with these more dispersible carbon nanotubes with the aim of making them easier to process. The creation of the smaller nanotubes is the first step toward reaching this goal.
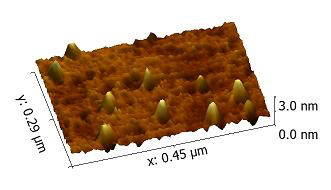
Illustration: Piles of zero-dimensional carbon nanotubes appear as gold “mountains” on a substrate by atomic force microscopy. The nanotube mountains are only a few nanometers high – or nearly a billion times smaller than an inch. – University of Pittsburgh Swanson School of Engineeering.
Integrating Mental Health Services in Pediatric Practices Feasible, Effective, Pitt Finds
 Brief behavioral and mental health programs for children can be effectively provided within pediatric practices as an alternative to being referred to a community specialist, University of Pittsburgh Schools of the Health Sciences researchers found in a National Institutes of Health-funded randomized trial. McGowan Institute for Regenerative Medicine affiliated faculty member Stephen Wisniewski, PhD, Associate Dean for Research in the Graduate School of Public Health, Executive Deputy Director in the Epidemiology Data Center, Associate Professor (secondary) in the Department of Psychiatry in the School of Medicine, Associate Professor in the Department of Epidemiology in the Graduate School of Public Health, and Guest Scientist in the Safer Center for Resuscitation Research, is a co-author on the study.
Brief behavioral and mental health programs for children can be effectively provided within pediatric practices as an alternative to being referred to a community specialist, University of Pittsburgh Schools of the Health Sciences researchers found in a National Institutes of Health-funded randomized trial. McGowan Institute for Regenerative Medicine affiliated faculty member Stephen Wisniewski, PhD, Associate Dean for Research in the Graduate School of Public Health, Executive Deputy Director in the Epidemiology Data Center, Associate Professor (secondary) in the Department of Psychiatry in the School of Medicine, Associate Professor in the Department of Epidemiology in the Graduate School of Public Health, and Guest Scientist in the Safer Center for Resuscitation Research, is a co-author on the study.
Behavioral health treatment provided in the pediatrician’s office resulted in improved access to care, greater participation by both the child and their caregiver in treatment programs, and higher rates of treatment completion, without burdening the pediatric practice, researchers report in the journal Pediatrics.
“Treating both physical and behavioral health in the office of the child’s pediatrician is an achievable goal that provides many benefits to the child, caregiver, and pediatrician,” said lead author David Kolko, PhD, Professor of Psychiatry, Psychology, Pediatrics, and Clinical and Translational Science in Pitt’s School of Medicine. “When the behavioral health treatment was provided in the pediatrician’s office, participants were more than six times as likely to complete the program, as they were when it was provided at a specialty care clinic outside the pediatrician’s office.”
Dr. Kolko and his colleagues recruited more than 300 children and their caregivers at eight community pediatric practices affiliated with Children’s Hospital of Pittsburgh of UPMC who had been referred for treatment of behavioral problems, though many also had attention-deficit/hyperactivity disorder (ADHD) or anxiety. This study is the third one completed by Services for Kids in Primary-Care (SKIP), a program that integrates behavioral health services into primary pediatric and family medicine practices.
In this trial, half the children received “doctor office collaborative care,” where a trained behavioral health clinician, known as a care manager, collaborated with the child’s pediatrician to deliver mental health services in the pediatrician’s office. The other half received “enhanced usual care,” where the patients received educational materials and were referred to a local mental health specialist outside the pediatrician’s office who accepted the child’s health insurance.
In both the in-office and outside specialist programs, the pediatrician was updated on the patient’s care and could prescribe medication for the child when necessary.
Of the participants assigned to the care manager at the pediatrician’s office, 99.4 percent began treatment programs and 76.6 percent completed them. Of those assigned to a specialist outside the office, 54.2 percent began treatment and 11.6 percent completed it.
“In fact, the participating pediatric practices in this clinical trial later hired their own mental health clinicians to continue delivering on-site services, after the trial had ended,” said Dr. Kolko. “Still more research is needed to understand how pediatric practices adapt clinical and financial strategies to make an in-office behavioral health provider a sustainable resource. Perhaps pediatricians who observe the program in operation may be willing to find a way to support these resources and make that service work.”
AWARDS AND RECOGNITION
Dr. Freddie Fu: National and International Honors
 McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, received the Elizabeth Winston Lanier Award for his career contribution to anterior cruciate ligament (ACL) reconstruction and advances in patient care, an accolade considered the Nobel Prize of orthopaedic research and bestowed by the Kappa Delta Sorority along with the Orthopaedic Research and Education Foundation. It was presented to Dr. Fu in New Orleans at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS), a conference of 10,000 professionals.
McGowan Institute for Regenerative Medicine faculty member Freddie Fu, MD, received the Elizabeth Winston Lanier Award for his career contribution to anterior cruciate ligament (ACL) reconstruction and advances in patient care, an accolade considered the Nobel Prize of orthopaedic research and bestowed by the Kappa Delta Sorority along with the Orthopaedic Research and Education Foundation. It was presented to Dr. Fu in New Orleans at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS), a conference of 10,000 professionals.
Dr. Fu, the David Silver Professor and Chairman of the University of Pittsburgh Department of Orthopaedic Surgery and founder of the UPMC Center for Sports Medicine, also will receive honors in Europe and Asia over the next 4 months:
In Amsterdam in May, the European Society of Sports Traumatology, Knee Surgery and Arthroscopy (ESSKA) is scheduled to present its highest honor to Dr. Fu by making him a Lifetime Honorary Member and a member of its Hall of Fame — the first from Pennsylvania, the second from the United States, and only the fourth inductee to date.
In Hiroshima, Japan, in July, the Japanese Orthopaedic Society of Knee, Arthroscopy and Sports Medicine (JOSKAS) will make Dr. Fu only the fourth surgeon — and second from the Western Hemisphere — recognized with the Masaki Watanabe Award for international achievement in arthroscopic surgery.
The Lanier Award, one of two Kappa Delta research awards established in 1947, honored Dr. Fu for his work surrounding ACL repair — an injury that can result in acute instability and long-term clinical problems, such as osteoarthritis. He supervised research that redefined long-standing assumptions about the ligament and developed new approaches to reconstructing it by replicating the knee’s native anatomy to restore long-term knee function, not to mention the patient’s quality of life.
On May 16 in The Netherlands, ESSKA will honor Dr. Fu at its General Assembly, which is held every other year. Its eight-member board of directors met in Glasgow, Scotland, last month and unanimously voted to award him with Lifetime Honorary Membership at its upcoming conference. ESSKA, with 4,000 members, is the largest orthopaedic society in Europe.
On July 26 in Japan, Dr. Fu will receive the Watanabe Award from JOSKAS, an organization founded 40 years ago, and its congress president, Dr. Mitsuo Ochi, director of Hiroshima University Hospital and chairman of the Department of Orthopaedic Surgery in the Graduate School of Biomedicine and Health Sciences at Hiroshima University.
Congratulations, Dr. Fu!
Provost Inaugural Lecture: Dr. David Perlmutter
 In celebration of his appointment, McGowan Institute for Regenerative Medicine affiliated faculty member David Perlmutter, MD, Distinguished Professor of Pediatrics, School of Medicine, University of Pittsburgh, presented the April 22, 2014, Provost Inaugural Lecture. His presentation was entitled, “The Role of Autophagy in Alpha-1-Antitrypsin Deficiency: Evolving in Pittsburgh.”
In celebration of his appointment, McGowan Institute for Regenerative Medicine affiliated faculty member David Perlmutter, MD, Distinguished Professor of Pediatrics, School of Medicine, University of Pittsburgh, presented the April 22, 2014, Provost Inaugural Lecture. His presentation was entitled, “The Role of Autophagy in Alpha-1-Antitrypsin Deficiency: Evolving in Pittsburgh.”
Dr. Perlmutter is the Vira I. Heinz Endowed Chairman of Pediatrics at the University of Pittsburgh School of Medicine. He is also a Professor of Cell Biology and Physiology in the School of Medicine. Additionally, Dr. Perlmutter is the Physician-in-Chief and Scientific Director of Children’s Hospital of Pittsburgh of UPMC.
Dr. Perlmutter is the author/co-author of many publications for his most recent research regarding disorders of infancy, childhood, and adolescence. Primarily, his efforts have focused on liver, lung, and gastroenterological diseases. The projects in which Dr. Perlmutter is currently involved include the following:
- New therapies for liver fibrosis and hyperproliferation in Alpha1-AT deficiency
- Novel pharmacological strategies for liver disease in antitrypsin deficiency
- Pathogenesis of hepatocellular carcinoma in antitrypsin deficiency
- Molecular basis of pediatric diseases
- Basic/translational research training for CHP pediatric training for CHP pediatric fellows
Dr. Anna Balazs Named 2014 ACS Langmuir Lecturer
 McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, the Robert v. d. Luft Distinguished Professor in Chemical and Petroleum Engineering, University of Pittsburgh Swanson School of Engineering, has been named the 2014 American Chemical Society (ACS) Langmuir Lecturer.
McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, the Robert v. d. Luft Distinguished Professor in Chemical and Petroleum Engineering, University of Pittsburgh Swanson School of Engineering, has been named the 2014 American Chemical Society (ACS) Langmuir Lecturer.
The Langmuir Lecture is quite distinguished and was first presented in 1979. The Lecturers are chosen from the international scientific community based on their outstanding research, publications, and creativity in the interdisciplinary field of Colloid and Surface Chemistry.
Dr. Balazs will deliver a plenary lecture in a special session of the Colloid and Surface Chemistry Division program, presented in the 2014 Fall ACS Meeting to be held in Indianapolis, Indiana.
Pitt/MWRI Researchers Awarded Prestigious Cozzarelli Prize for Top Biomedical Sciences PNAS Paper of 2013
 Researchers at the University of Pittsburgh School of Medicine and Magee-Womens Research Institute (MWRI) have been awarded the Cozzarelli Prize in the biomedical sciences for a July 2013 paper published in the Proceedings of the National Academy of Sciences (PNAS) that showed the cells of the placenta may have a unique ability to prevent viruses from crossing from an expectant mother to her growing baby and can transfer that trait to other kinds of cells.
Researchers at the University of Pittsburgh School of Medicine and Magee-Womens Research Institute (MWRI) have been awarded the Cozzarelli Prize in the biomedical sciences for a July 2013 paper published in the Proceedings of the National Academy of Sciences (PNAS) that showed the cells of the placenta may have a unique ability to prevent viruses from crossing from an expectant mother to her growing baby and can transfer that trait to other kinds of cells.
Senior authors Yoel Sadovsky, MD, Elsie Hilliard Hillman Chair of Women’s Health Research, professor of obstetrics, gynecology and reproductive medicine, Pitt School of Medicine, and MWRI director, and Carolyn Coyne, PhD, associate professor, Department of Microbiology and Molecular Genetics at Pitt, and MWRI member, and their research team will be honored on April 27 at a ceremony in Washington, DC. McGowan Institute for Regenerative Medicine faculty member Donna Stolz, PhD, associate director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and associate professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is also a co-author on the study.
The annual award was established in 2005 and named in 2007 for late PNAS Editor-in-Chief Nicholas R. Cozzarelli and acknowledges papers that reflect scientific excellence and originality. Five other papers in fields including the physical and mathematical sciences; biological sciences; engineering and applied sciences; behavioral and social sciences; and applied biological, agricultural, and environmental sciences that were published in PNAS in 2013 also will receive awards.
For their paper, which was published on July 16, 2013, the research team studied human trophoblast cells in the lab, exposing them to a panel of viruses. Unlike non-placental cells, trophoblasts were resistant to viral infection, but that trait was not a result of an inability of viruses to bind or enter the cells. When the medium, or fluid environment, in which the trophoblasts were cultured was transferred to non-placental cells, such as those that line blood vessels, they became resistant to viral infection, too.
The team found that when the medium was exposed to sound waves in a process called sonication, viral resistance was no longer transferred to non-placental cells. This finding led them to take a closer look at exosomes, which are tiny spheres called nanovesicles that are secreted by trophoblasts and are sensitive to sonication. Fragments of genetic material called microRNAs contained within the exosomes, as well as lab-synthesized mimics of them, were able to induce autophagy, a mechanism of prolonged cellular recycling and survival. Blocking autophagy at least partially restored the cells’ vulnerability to viral infections.
 Regenerative Medicine Podcast Update
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#134 – Dr. Herbert Ray an Assistant Professor of Endodontics and the Director, Graduate Endodontic Residency Program, University of Pittsburgh School of Dental Medicine. Dr. Ray discusses his research on dental pulp regeneration.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
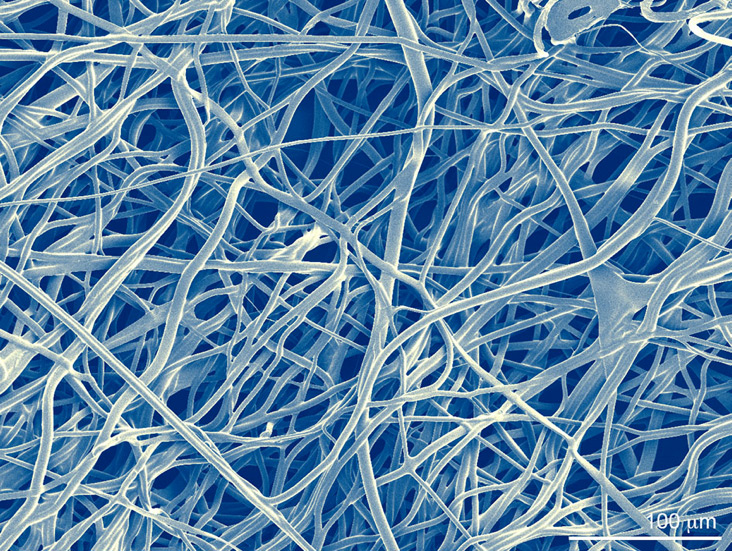
Engineering porosity into micro fibrous scaffolds, Scanning Electron Microscopy.
Image by Robert A. Allen
AHA Predoctoral Fellow and BICP Chair
Biomaterials Foundry / Yadong Wang Laboratory
Department of Bioengineering, University of Pittsburgh
We welcome submissions from others for future Pictures of the Month.

