March 2018 | VOL. 17, NO. 3| www.McGowan.pitt.edu
McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat

Photo by Michael Drazdzinski
The McGowan Institute for Regenerative Medicine held its 2018 Scientific Retreat March 5-6, 2018. The focus was on peer-to-peer networking, and the retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful discussions and identification of opportunities for partnerships. This year’s program committee under the leadership of Julie Phillippi, PhD, Patrick Cantini, and Christopher Bettinger, PhD, designed an exciting program.
Program Highlights

Photo by Michael Drazdzinski
The Director’s Welcome and State of the Institute Address was presented by McGowan Institute Director William Wagner, PhD, Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. Dr. Wagner followed his presentation with an introduction of the McGowan Institute for Regenerative Medicine Distinguished Lecturer, Molly Shoichet, PhD, University Professor, Program of Neuroscience, University of Toronto. Dr. Shoichet’s presentation was entitled “Regenerative Strategies in the Central Nervous System.”
The Retreat included numerous general sessions throughout the 2-day event with a focus on these research and/or strategic planning areas:
- Cell and Tissue Regenerative Therapies at UPMC
- Reproductive Tissue Engineering and Women’s Health
- Vision Restoration
- Nano- and Microfabrication Methods for Medical Applications
- Advancing Your Early-Stage Research Toward Commercial Translation
- Stem Cells and Aging
- Translating Redox Vascular Biology to the Clinic
- Craniomaxillofacial Tissue Engineering
- Pittsburgh Biomedical Breakfast
- Opening Doors to the Future of Cardiovascular Regenerative Medicine
- Translating the Science of Tissue Plasticity for Human Performance Optimization and Injury Prevention
- Cancer Immunotherapy
- Pediatric Innovations
- Additive Manufacturing
- Medical Technology Development—From Prototype to FDA Approval
- Sponsored Project Research Overview—Successful Submission and Post Award Management
- Pitt is Open for Business
- Innovations in Clinical Diagnostics and Biomarkers
- Drug Delivery
- Strategic Planning Session—Optimizing Human
- Materials and Systems Performance
The Retreat program also included 91 presentations addressing a great cross-section of scientific topics given by McGowan Institute affiliated faculty and 59 invited researchers from other institutions and agencies who are not formally affiliated with the McGowan Institute (in alphabetical order):
- Esta Abelev, PhD, Technical Director, Petersen Institute of Nanoscience and Engineering, University of Pittsburgh: Nano-and Microfabrication Methods for Medical Applications (session organizer)
- Salah Al-Zaiti, PhD, RN, ANP-BC, Assistant Professor, Acute & Tertiary Care, School of Nursing, University of Pittsburgh: ECG Method to Detect Non-ST Cardiac Events
- Michael Annichine, Chief Executive Officer, Magee-Womens Research Institute and Foundation: Magee-Womens Research Institute’s Scientific Entrepreneurship and Its Impact
- Edgar Aranda-Michel, BS, MD/PhD Candidate, Medical Scientist Training Program (MSTP), University of Pittsburgh and Carnegie Mellon University: Novel Biological Muscle Powered Heart Assist Device
- Amanda Artsen, MD, Graduate Medical Trainee, University of Pittsburgh: Mesh for Pelvic Organ Prolapse Repair: Optimizing Biomechanics and Exploring the Immune Response
- Christian Barrow, Executive Director of Life Sciences, JP Morgan: Comments on the 2018 JP Morgan Healthcare Conference
- Rachel Berger, MD, MPH, Child Protection Team, Pittsburgh Child Advocacy Center, Children’s Hospital of Pittsburgh of UPMC; and Director, Child Abuse Research, Safar Center for Resuscitation Research, University of Pittsburgh: The Biomarkers for Infant Brain Injury Score (BIBIS): Improving Identification of Intracranial Hemorrhage in Infants and Young Children Using Serum Biomarkers
- Michael Buckenmeyer, MS, PhD Candidate, University of Pittsburgh: Regenerative Therapies for Medically Induced Infertility
- Amin Cheikhi, PhD, Postdoctoral Assignment, School of Medicine, University of Pittsburgh: Mitochondrial Architecture as Read-Out for Cell Memory and Aging
- Bill Chen, PhD, Director, Small Molecule Therapeutic Center Co-Director, Acute Lung Injury Center of Excellence, Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh: Targeting Fbxo48/AMPK Axis in Metabolic Diseases
- Paola Corti, PhD, Research Assistant Professor, Division of Cardiology Vascular Medicine Institute, University of Pittsburgh: Globin X, Nitrite and Heart Regeneration
- Noel Jabbour, MD, MS, FACS, Assistant Professor, Department of Otolaryngology; Fellowship Director, Division of Pediatric Otolaryngology; Associate Director, Otolaryngology Residency Program; Director, Congenital Ear Center, University of Pittsburgh: Born Without an Ear: Current and Future Solutions for Congenital Auricular Deformities
- Juan Taboas, PhD, Assistant Professor, Department of Oral Biology, School of Dental Medicine, Department of Biomedical Engineering, Swanson School of Engineering, University of Pittsburgh: Vital-Dent
- Heather Szabo-Rogers, PhD, Assistant Professor, Department of Oral Biology, Center for Craniofacial Regeneration, School of Dental Medicine, University of Pittsburgh: Lessons from Developmental Biology toward Regeneration
- Fatima Syed-Picard, PhD, Assistant Professor, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh: Dental Stem Cells and Scaffold-free Tissue Engineering for Craniofacial Regeneration
- Garrett Coyan, MD, Integrated 6-year Residency Program (PGY4)-Department of Cardiothoracic Surgery, University of Pittsburgh Medical Center: Acute In Vivo Assessment of Tissue Engineered Heart Valves: Early Successes and Challenges
- Julie Cramer, PhD, MS, Commercialization Translation Program Manager, sciVelo, University of Pittsburgh: Innovations in Clinical Diagnostics and Biomarkers (co-chair) and Cancer Immunotherapy (session co-organizer)
- Diwikar Davar, MBBS, MSc, Assistant Professor of Medicine, Division of Hematology/ Oncology, Department of Medicine, University of Pittsburgh: Novel Targets in Immunotherapy for Cancer
- Evan Delgado, PhD, Post-Doctoral Researcher, Department of Cellular and Molecular Pathology, University of Pittsburgh: Innovations in Clinical Diagnostics and Biomarkers (session co-chair)
- Terence Dermody, MD, Vira I. Heinz Professor and Chair, Department of Pediatrics, Children’s Hospital of Pittsburgh; and Professor of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, Physician-in-Chief and Scientific Director, Children’s Hospital of Pittsburgh of UPMC: New Research Initiatives at Children’s Hospital of Pittsburgh of UPMC
- Alex Ducruet, PhD, CLP: Patenting Process at Pitt
- Leonid Emerel, MD, Integrated 6-year Residency Program (PGY5)-Department of Cardiothoracic Surgery, University of Pittsburgh: Biomechanical Properties and Stress Mapping Dissection Probability Models in Ascending Aortas of Patients Sustaining Acute Type A Aortic Dissection
- Max Fedor: How to Find Start-Up Funding
- Toren Finkel, MD, PhD, Professor of Medicine, Division of Cardiology, G. Nicholas Beckwith III and Dorothy B. Beckwith Chair in Translational Medicine; Director, Aging Institute of UPMC and University of Pittsburgh: Mitochondria, Aging and Stem Cell Function
- Olivera Finn, PhD, Distinguished Professor, Departments of Immunology and Surgery, University of Pittsburgh School of Medicine: Vaccines for Cancer Prevention and A Lot More
- Shawn Flanagan, PhD, Assistant Professor, Department of Sports Medicine and Nutrition, University of Pittsburgh: Neurobiology Plasticity: Corticospinal Basis of Persistent Functional Deficits after Traumatic Musculoskeletal Injury
- Aditi Gurkar, PhD, Assistant Professor of Medicine, Division of Geriatric Medicine, Department of Medicine, University of Pittsburgh: Endogenous DNA Damage Drives Dilated Cardiomyopathy
- Jeffrey Gusenoff, MD, Department of Plastic Surgery, University of Pittsburgh Medical Center: Adipose Therapy for Foot Pain: A New Paradigm for Treating a Difficult Problem Translated to the Clinic
- J. Hinton, PhD, Postdoctoral Fellow, Department of Biomedical Engineering, Materials Science & Engineering, Carnegie Mellon University: 3D Bioprinting of Soft and Living Materials Using Open Source Hardware
- Deborah Hollingshead, MS: Increasing Data Reproducibility
- Michael Hufford, PhD, Vice President for Regulatory Affairs, Behavioral Science, and Innovation, Pinney Associates: Citius, Altius, Fortius: Drug Delivery and the Generation, and Destruction, of Value in Drug Development
- Julie Hughes, PhD, Research Physiologist, U.S. Army Research Institute of Environmental Medicine: Bone Plasticity: Exploiting the Adaptive Bone Formation Process to Prevent Stress Fractures in Warfighters
- Theodore Huppert, PhD, Associate Professor, Departments of Radiology and Bioengineering, University of Pittsburgh: Neuromonitoring After Brain Injury in Pediatrics
- Noel Jabbour, MD, MS, FACS, Assistant Professor, Department of Otolaryngology; Fellowship Director, Division of Pediatric Otolaryngology; Associate Director, Otolaryngology Residency Program; Director, Congenital Ear Center, University of Pittsburgh: Born Without an Ear: Current and Future Solutions for Congenital Auricular Deformities
- Ashu Jadhav, MD, PhD, Assistant Professor, Departments of Neurology and Neurosurgery, University of Pittsburgh Medical Center: Regenerative Cell Therapy for Brain Injury
- Katrina Knight, PhD, Department of Medicine, University of Pittsburgh: Mesh for Pelvic Organ Prolapse Repair: Optimizing Biomechanics and Exploring the Immune Response
- Takaaki Kuwajima, PhD, Research Assistant Professor, Department of Ophthalmology, University of Pittsburgh School of Medicine: Dissecting Molecular Mechanisms of Retinal Axon Degeneration and Regeneration
- Bill LaFramboise, PhD, Associate Professor, Department of Pathology, University of Pittsburgh Medical Center: Bladder Cancer Biomarkers
- Janette Lamb, PhD: Increasing Data Reproducibility
- Chris Mahoney, MS, PhD Candidate, University of Pittsburgh: Tissue Engineering Strategies for Breast Cancer Reconstruction
- Christian Manders, Promethean LifeSciences, Inc.: Pittsburgh Biomedical Breakfast (session organizer)
- Mioara Manole, MD, FAAP, Director of Basic and Translational Research, Division of Pediatric Emergency Medicine, Children’s Hospital of Pittsburgh of UPMC; Associate Director, Safar Center for Resuscitation Research; and Assistant Professor of Pediatrics, University of Pittsburgh School of Medicine: Neuromonitoring After Brain Injury in Pediatrics
- Quyen Nguyen, MD, Associate Professor, Fellow, Pulmonary & Critical Care Medicine, UPMC Montefiore Hospital: Platelet Mitochondrial Bioenergetics in Pulmonary Hypertension
- Nick Nystrom, Director, Pittsburgh Supercomputing Center: Unleashing AI and Data for Medicine
- Kentaro Onishi, DO, Associate Faculty, Assistant Professor Departments of Physical Medicine and Rehabilitation and of Orthopaedic Surgery, University of Pittsburgh: Regenerative Cell Therapy for Sports Medicine and Orthopedic Problems: Opportunities and Challenges
- Kyle Orwig, PhD, Professor, Department of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh: Regenerative Therapies for Medically Induced Infertility
- Amy Phillips, MBA, Director, Office of Corporate Engagement, University of Pittsburgh: Pitt is Open for Business
- Ian Pollack, MD, Co-director, Brain Tumor Program, University of Pittsburgh Cancer Institute; Chief, Pediatric Neurosurgery, Children’s Hospital of Pittsburgh of UPMC; and A. Leland Albright Professor of Neurosurgery, University of Pittsburgh School of Medicine: Peptide-Based Immunotherapy for Childhood Gliomas
- Margaret Prendergast, Director of Bioengineering, Allevi: Allevi–Automating Biology
- Abhijit Roy, PhD, Research Assistant Professor, Department of Bioengineering, University of Pittsburgh: Additive Manufacturing Approaches to Resorbable Metals, Ceramics and Polymers
- George Santo, Vice President, Middle Market Life Sciences Banking, JP Morgan: Comments on the 2018 JP Morgan Healthcare Conference
- Ben Schilling, MS, PhD Candidate, University of Pittsburgh: Tissue Engineering Strategies for Breast Cancer Reconstruction
- Francisco Schopfer, PhD, Associate Professor, Department of Pharmacology and Chemical Biology, University of Pittsburgh: Nitrated Fatty Acids: The Road from Discovery to the Clinic
- Brian Smith, Senior Financial Analyst, McGowan Institute for Regenerative Medicine: Sponsored Project Research Overview: Successful Submission and Post Award Management
- Nick Smith, Graduate Student Researcher, Department of Pharmacology and Chemical Biology, University of Pittsburgh: Breast Cancer Genetic Profiling
- Lorenzo Soletti, PhD: How to Start a Company
- Adam Straub, PhD, Assistant Professor, Department of Pharmacology and Chemical Biology; and Principal Investigator, Vascular Medicine Institute, University of Pittsburgh: Heme Redox Regulation and NO Signaling
- Eric Sugalski, President and Founder, Smithwise: Medical Technology Development: From Prototype to FDA Approval (session organizer)
- Stephen Thorne, PhD, Assistant Professor, Departments of Surgery and Immunology, University of Pittsburgh School of Medicine; and Leader of Small Animal Optical and Ultrasound Imaging Services In Vivo Imaging Facility, University of Pittsburgh Cancer Institute: Developing Multi-Mechanistic Cancer Immunotherapies, Experiences in Academia and Industry
- Brian Vidic, Executive Director, Research Operations and Business Engagement Office, University of Pittsburgh: Pitt is Open for Business
- Theresa Whiteside, PhD, Professor of Pathology, Immunology, and Otolaryngology, University of Pittsburgh School of Medicine; and Laboratory Director of Immunologic Monitoring and Cellular Products Laboratory, University of Pittsburgh Cancer Institute: Tumor-Derived Exosomes as Potential Biomarkers in Cancer
- Eunice Yang, PhD, Assistant Professor, Department of Mechanical Engineering, University of Pittsburgh at Johnstown: Orthostatic Hypotension Detection to Predict Falls
- Luke Ziegler, BS, Hemorheology, Hemodynamics and Artificial Blood Research Laboratory, McGowan Institute for Regenerative Medicine, University of Pittsburgh: Sickle Cell Hemoglobin Replacement Therapy
The closing banquet included three excellent presentations. They included:
“Reflections on the Patient Experience in Respiratory Failure”
Jonathan D’Cunha, MD, PhD, FACS
Associate Professor of Surgery Department of Cardiothoracic Surgery; Vice Chair, Research and Education; and Chief, Division of Lung Transplant/Lung Failure University of Pittsburgh
“An Accidental Entrepreneur’s Journey Toward Portable Respiratory Assist Devices”
William J. Federspiel, PhD
William Kepler Whiteford Professor Department of Bioengineering University of Pittsburgh; and Director, Medical Devices Laboratory McGowan Institute for Regenerative Medicine
“Keynote Presentation: Pittsburgh as the Future of Health Care—The Role of the Local Biomedical Research Ecosystem”
Steven Shapiro, MD
Executive Vice President Chief Medical and Scientific Officer President, Health Services Division University of Pittsburgh Medical Center
Poster Session
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
Cell and Gene Therapy
First Place
Patrick Wilkinson
A role for polyploid hepatocytes in liver repopulation and adaptation to chronic injury
Mentor: Andrew Duncan, PhD
Dept. of Pathology, McGowan Institute
University of Pittsburgh
Second Place
Matthew Rannals
Targeted translatome profiling using in utero gene transfer identifies ion channel pathophysiology in a brain development model of transcription factor 4 (TCF4) function
Mentor: Nathan Urban, PhD
Dept. of Neurobiology
University of Pittsburgh
Third Place
Aaron Sun
Differences in Neural Stem Cell Identity and Differentiation Capacity Modulate Divergent Regenerative Outcomes in Lizards and Salamanders
Mentors: Thomas Lozito, PhD; Rocky Tuan, PhD
Dept. of Orthopaedic Surgery, McGowan Institute
University of Pittsburgh
Computation and Modeling

Photo by Aimee Colabine Beattie
First Place
Ehab Tamimi
Computationally optimizing the compliance of biopolymer tissue engineered vascular grafts
Mentor: Jonathan Vande Geest, PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Second Place
Reza Behkam
The aorta and vocal fold paralysis: is there a connection?
Mentor: Jonathan Vande Geest, PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Third Place
Lukas Schimunek
Controllability Analysis of Protein-Protein Interaction Networks for Antiviral Drug Development
Mentor: Yoram Vodovotz, PhD
Dept. of Surgery, McGowan Institute
University of Pittsburgh
Medical Devices
First Place
Firuz Feturi
Enzyme Responsive-Release Hydrogel for Targeted Immunosuppression in Vascularized Composite Allotransplantation
Mentor: Vijay Gorantla, MD, PhD
Dept. of Plastic and Reconstructive Surgery, McGowan Institute
University of Pittsburgh
Second Place
Aimon Iftikhar
A Clinically Relevant Rabbit Surgical Model of Pelvic Reconstruction to Evaluate the Regenerative Immune Response to Novel Surgical Materials
Mentor: Bryan Brown, PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Third Place
Eoghan Cunnane
Developing urinary catheter safety devices and improving tissue engineered urethral scaffolds through an enhanced understanding of human urethral biomechanics
Mentors: Michael Walsh, PhD; David Vorp, PhD
School of Engineering, Bernal Institute, University of Limerick
Dept. of Bioengineering, McGowan Institute, University of Pittsburgh
Tissue Engineering
First Place
Sahil Rastogi
Graphene-based Biocompatible and Transparent Micro-Electrode Arrays for Simultaneous Electrical and Optical Measurements of Electrogenic Cells
Mentor: Tzahi Cohen-Karni, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Second Place
Lindsey Saldin
Extracellular Matrix Hydrogel Downregulates Neoplastic Esophageal Cell Phenotype
Mentor: Stephen Badylak, DVM, PhD, MD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Third Place
Harman Ghuman
ECM hydrogel injection for the treatment of stroke: Time course comparison of hydrogel retention and phenotypic characterization of invading cells
Mentor: Mike Modo, PhD
Dept. of Radiology, McGowan Institute
University of Pittsburgh
People’s Choice (inaugural award this year)
First Place
Vishal Rakshe
Shape Dependent Effects of Nanomaterials on Macrophage Polarization
Mentor: Shilpa Sant, PhD
Dept. of Pharmaceutical Science, McGowan Institute
University of Pittsburgh
Second Place
Akhil Patel
RegenMatrix: A Growth Factor-free Bone Graft for Craniofacial Bone Regeneration
Mentor: Shilpa Sant, PhD
Dept. of Pharmaceutical Science, McGowan Institute
University of Pittsburgh
Third Place
Karis Kosar
Treatment of a Mouse Model of Cholestasis with a Thyromimetic Improves Biliary Injury but Exacerbates Hepatocyte Injury
Mentor: Kari Nejak-Bowen, PhD, MBA
Dept. of Pathology, McGowan Institute
University of Pittsburgh
Poster Awards Sponsored by sciVelo
First place: $200 (each category)
Second place: $125
Third place: $75
CATER Program

Photo by Aimee Colabine Beattie
First Place
Aaron Sun
Differences in Neural Stem Cell Identity and Differentiation Capacity Modulate Divergent Regenerative Outcomes in Lizards and Salamanders
Mentors: Thomas Lozito, PhD, and Rocky Tuan, PhD
Dept. of Orthopaedic Surgery, McGowan Institute
University of Pittsburgh
Second Place
Colin Beckwitt
Adjuvant Statin Therapy Efficacy is dictated by Tumor Dormancy and Statin Lipophilicity in ex vivo and in vivo Models of Metastatic Breast Cancer
Mentor: Alan Wells, MD, DMS
Dept. of Pathology
University of Pittsburgh
Third Place
Jacquelyn Russell
Lack of Beta-catenin in Hepatocytes Impairs Proliferation and Promotes Liver Progenitor Cell-Mediated Repair in Response to the Choline-Deficient Ethionine-Supplemented Diet
Mentor: Paul Monga, MD
Dept. of Pathology
University of Pittsburgh
Awards by CATER
First place: $200
Second place: $125
Third place: $75
A special thank you is extended to all who made this year’s Retreat a success!
See the full Retreat Program here.
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special
Old Man Winter has packed his bags and headed North for a well-deserved vacation!
Before we say goodbye to the chilly thrills of winter, let’s have one more month of frozen fun! Bring your frozen tissue to the McGowan Lab for 30% off frozen tissue sectioning and 30% off our beautiful Oil Red O staining!

Oil Red O (Lipid Stain) is intended for use in the histological visualization of fat cells and neutral fat. The staining has to be performed on fresh samples, as alcohol fixation removes most lipids.
The McGowan Histology Lab is equipped with state of the art processing, embedding, and staining automation, including instruments for paraffin and frozen sections alike. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the lowest length of time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Flow Cytometry at Work – Seeking What Influences the Pathogenesis of Fibrosis
Research teams from the labs of McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, Assistant Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh, and affiliated faculty member Cecelia Yates, PhD, Assistant Professor, Department of Health Promotion & Development, School of Nursing, University of Pittsburgh, with a secondary appointment in the Department of Pathology, School of Medicine, recently used flow cytometry to aid in their research into the pathogenesis of fibrosis. The teams’ experience using flow cytometry in their studies follows:
Fibrotic diseases are associated with 45% of deaths in the United States, but effective therapy does not exist, representing a significant unmet medical need. While the etiology of fibrosis is complex and multifactorial, some pathological features are common irrespective of cause. Drs. Brown and Yates have forged a collaborative pursuit to dissect the cell-cell communication between fibroblasts and macrophages that leads to the development of pathological fibrosis. The Brown lab focuses on a macrophage-centered approach, and the Yates lab focuses on a fibroblast-extracellular matrix driven approach to understand the underlying causes of fibrosis and healing. In a multi-disciplinary fashion, using their combined expertise, the Brown-Yates labs have begun to elucidate the precise functions of fibroblasts (master producers of ECM) and macrophages (master regulators of ECM production) in the bi-directional crosstalk that influences the pathogenesis of fibrosis. The aim is to determine if one cell type acts as a master coordinator and, ultimately, how this crosstalk can be leveraged to inhibit fibrotic diseases.
These research efforts are led by post-doctoral fellow Zariel Johnson, PhD (Yates), and graduate student Samuel LoPresti (Brown). The team consulted extensively with Lynda Guzik, McGowan Institute Flow Cytometry Laboratory Manager, to design appropriate flow cytometry protocols and methods to address these complex questions.
The primary research question required a thoughtful and comprehensive plan. Ms. Guzik committed herself from the start. She guided our research team at all stages of our experiments, from the design of our ex vivo experiments for evaluating cell-contact-independent crosstalk all the way to interpretation of the results. She took a particular interest in the design of marker panels, that were multicolored, to profile the heterocellular phenotypes and expression patterns. Naturally, this resulted in the requirement for sophisticated analyses, and Ms. Guzik methodically and meticulously guided the interpretation of the results.
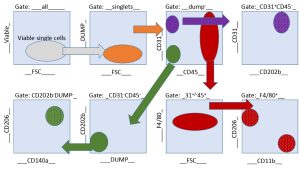
There was no challenge Ms. Guzik shied away from in our project. We were interested in understanding the single cell gene-alterations during fibrotic healing. This required FACS sorting to sort several cell types from wound tissue biopsies and for downstream RNA analysis. Ms. Guzik designed an elegant gating strategy to sort four different cell populations. (See sample gating strategy for endothelial cells, fibroblasts, M1 and M2 macrophages cell sorting diagram.)
Being able to utilize Ms. Guzik’s expertise at the Flow Cytometry Laboratory is an incredible asset and one that certainly enhanced our research efforts.
If you have a research project where flow cytometry may benefit your efforts, please feel free to contact Lynda Guzik for more information.
UPCOMING EVENTS
 Regenerative Medicine Summer School 2018
Regenerative Medicine Summer School 2018
This year marks the 5th Annual McGowan Institute for Regenerative Medicine Summer School. This program is open to undergraduate students from all backgrounds, and serves as an in-depth introduction to regenerative medicine. Students attend lectures and have the opportunity to interact with more than 20 faculty, spanning highly diverse disciplines including basic science, engineering, medicine, clinical translation, and university start-ups and established biomedical industry. Students also participate in hands-on laboratory activities within the laboratories of the McGowan Institute, participate in networking opportunities, and visit multiple Pittsburgh sites. This year, there will also be 8-10 week paid internships available for interested students. Students will participate in the week-long Summer School program, and then continue to perform deeper research with a faculty member of the McGowan Institute. We hope that these opportunities will spur interest in the field of regenerative medicine and result in increased participation of students from diverse backgrounds. Interested students should visit http://www.mirm-pitt.net/professional-development/summer-school/. Faculty interested in hosting a student should contact Dr. Bryan Brown at brownb@upmc.edu.
7th Annual Internal Symposium on Regenerative Rehabilitation Symposium

The Symposium on Regenerative Rehabilitation is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world, bringing together two disparate disciplines, regenerative medicine and rehabilitative research, into one synergistic field of science. Innovative ideas, concepts and technologies were shared, deliberated and debated at this premier event. This new and innovative approach combines discoveries in tissue engineering and cellular therapies with rehabilitative treatments, resulting in improved functional outcomes for patients. This Symposium encourages the participation of scientists, clinicians and physical therapists who are in the fields of regeneration, physical medicine and rehabilitation. The Symposium agenda is designed to create a platform for bridging these areas of expertise in a setting that fosters discussion, interaction, cross-discipline pollination and networking.
The Symposium will be held October 11-13, 2018 in Seattle, Washington.
SCIENTIFIC ADVANCES
UPMC Performs 1,000th Minimally Invasive Heart Valve Repair Procedure

Surgeons and interventional cardiologists at the UPMC Heart and Vascular Institute recently performed their 1,000th transcatheter aortic valve replacement (TAVR), making them the first heart program in the region to reach this clinical milestone.
The minimally invasive TAVR procedure is used to treat patients with aortic stenosis, a narrowing of the heart’s aortic valve that can lead to debilitating symptoms, such as shortness of breath, lightheadedness and fatigue or shortened lifespan.
During the procedure, an artificial valve is implanted through a catheter, which is inserted through a large artery in the patient’s leg or chest, eliminating the need for open-heart surgery. The procedure is performed in a specialized hybrid operating room that allows for maximum collaboration between surgeons and interventional cardiologists.
“Since beginning to perform the surgery in 2011, UPMC physicians continue to be one of the most innovative and experienced TAVR teams in the country,” said McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gleason, MD, chief of adult cardiac surgery at the UPMC Heart and Vascular Institute and co-director, Center for Aortic Valve Disease. “Not every patient is well-suited for open-heart surgery, and this procedure provides them with another option for aortic valve replacement, with the added benefits of a shorter hospital stay and a faster recovery.”
Previously, TAVR was used only if a patient was considered inoperable or high-risk for a traditional valve replacement, but the procedure recently received approval for intermediate-risk patients. TAVR also can be used to replace degenerating or failing bioprosthetic aortic valves, eliminating the need for those patients to have a second open-heart surgery.
“Aortic stenosis can interfere with daily activities as basic as walking,” said Dustin Kliner, MD, interventional cardiologist at the UPMC Heart and Vascular Institute. “This procedure can lengthen and greatly improve the quality of a patient’s life, getting them back to the activities that they enjoy most.”
Visit the UPMC Center for Aortic Valve Disease website for more information about aortic stenosis and the UPMC TAVR program.
Preventing Exhaustion in Immune Cells Boosts Immunotherapy in Mice

If you’re an immune cell gearing up to fight cancer, you’d better eat your breakfast. The tumor microenvironment is a harsh place, and tumor cells are ready to wear you out.
Cancer immunotherapies that improve the ability of T-cells – highly specialized immune soldiers – to attack cancer have made major strides in the clinic. However, they work only for 10 to 30 percent of cancer patients. One reason for this is a phenomenon called “T-cell exhaustion,” where the T-cells that are most specialized to kill cancer are continually stimulated, becoming drained of their energy due to the harsh conditions inside the tumor.
New research—co-authored by McGowan Institute for Regenerative Medicine affiliated faculty member Simon Watkins, PhD, founder and director of the Center for Biologic Imaging at the University of Pittsburgh, member of the Pittsburgh Cancer Institute, and Professor and vice chairman within the Department of Cell Biology—from the University of Pittsburgh School of Medicine and the UPMC Hillman Cancer Center shows that preventing or reversing this metabolic exhaustion with targeted therapies could enhance the effects of immunotherapy, potentially allowing them to help more patients.
The research, published recently in the Journal of Experimental Medicine, reveals this insight by uncovering how a protein on the surface of T-cells, called 41BB, works. 41BB is part of a family of “co-stimulators” normally activated when T-cells are fighting infections, but the conditions inside a tumor prevent this from occurring. Previous research has shown that activating 41BB helps T-cells replicate and persist long-term, but how it does that has remained a mystery.
“What we found is that 41BB’s effect can be almost completely chalked up to how it alters T-cell metabolism. In a sense, activating 41BB keeps the T-cells well fed so they last longer in a fight,” said Greg Delgoffe, PhD, assistant professor of immunology at Pitt’s School of Medicine and an investigator at the UPMC Immune Transplant and Therapy Center.
In lab-grown mouse T-cells, the researchers found that a protein antibody that activated 41BB caused its mitochondria – the cellular powerhouses – to both grow in number and fuse with each other.
“Activating 41BB increased the energy reserves of T-cells so they were ready to swiftly unleash their killing ability on demand,” said Ashley Menk, a researcher in Delgoffe’s lab and the first author of the study.
However, drugs that activate 41BB have not had much success on their own in clinical trials. To overcome this, the researchers tested whether 41BB activation, while not effective on its own, could improve the effect of two immunotherapy approaches: a checkpoint inhibitor drug that blocks the PD1 protein on T-cells and a cellular therapy that uses engineered T cells to recognize tumor cells.
When tested in a mouse model of melanoma that normally responds poorly to PD1 immunotherapy or therapeutic T cells on their own, the combination resulted in much better outcomes.
“While activating 41BB fixes the fuel issue, it doesn’t appear to fix the immunological issue, which is what checkpoint inhibitors or cellular therapies are great at. The combination of the two, we found, was better than the sum of its parts,” said Dr. Delgoffe.
Intriguingly, the team also found that pre-treating mice with a short duration of 41BB activation followed by PD1 immunotherapy was as good as using both drugs throughout, an approach that could potentially avoid side effects associated with long-term 41BB activation.
The researchers are testing the combination treatment in human tumor models and expect to conduct clinical trials in the near future.
Video Game Improves Doctors’ Recognition and Triage of Severe Trauma Patients

Playing an adventure video game featuring a fictitious, young emergency physician treating severe trauma patients was better than text-based learning at priming real doctors to quickly recognize the patients who needed higher levels of care, according to a new trial led by the University of Pittsburgh School of Medicine. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, Chair of the Department of Critical Care Medicine of both the University of Pittsburgh School of Medicine and the UPMC Healthcare System and a Distinguished Professor and the Mitchell P. Fink Endowed Chair in Critical Care Medicine with secondary appointments in Medicine, Health Policy and Management, and Clinical and Translational Science, was a member of the study team.
The results, published by The BMJ, held even though doctors assigned to the game enjoyed it less than those assigned to traditional, text-based education. This indicates that if game enjoyment can be improved, the already favorable results might be enhanced.
“Physicians must make decisions quickly and with incomplete information. Each year, 30,000 preventable deaths occur after injury, in part because patients with severe injuries who initially present to non-trauma centers are not promptly transferred to a hospital that can provide appropriate care,” said lead author Deepika Mohan, MD, MPH, assistant professor in Pitt’s departments of Critical Care Medicine and Surgery. “An hour of playing the video game recalibrated physicians’ brains to such a degree that, six months later, they were still out-performing their peers in recognizing severe trauma.”
Dr. Mohan created the game Night Shift with Schell Games, a Pittsburgh-based educational and entertainment game development company. The game is designed to tap into the part of the brain that uses pattern recognition and previous experience to make snap decisions using subconscious mental shortcuts – a process called heuristics.
Physicians in non-trauma centers typically see only about one severe trauma per 1,000 patients. As a result, their heuristic abilities can become skewed toward obvious injuries such as gunshot wounds, and miss equally severe traumas such as internal injuries from falls. On average, 70 percent of severely injured patients who present to non-trauma centers are under-triaged and not transferred to trauma centers as recommended by clinical practice.
Both the game and the text-based learning are intended to help physicians improve their decision making regarding severe traumas. The game, however, sought to do this through narrative engagement, or the use of stories to promote behavior change, which has shown promise in recalibrating heuristics.
Dr. Mohan’s team recruited 368 emergency medicine physicians from across the country who did not work at hospitals with trauma specialization. Half were assigned to play the game and half were asked to spend at least an hour reading the educational materials.
Participants then responded to questionnaires and completed a simulation that tested how often they “under-triaged,” or failed to send severe trauma patients to hospitals with the resources necessary to handle them. Physicians who played the game under-triaged 53 percent of the time, compared with 64 percent for those who read the educational materials.
Six months later, Dr. Mohan reassessed the physicians and found that the effect of the game persisted, with those who played the game under-triaging 57 percent of the time, compared to 74 percent for those who had read the educational materials.
“There are many reasons beyond the doctor’s heuristics as to why a severe trauma patient wouldn’t be transferred to a trauma center, ranging from not having an ambulance available to a lack of proper diagnostic tools,” said Dr. Mohan. “So, it is important to emphasize that recalibrating heuristics won’t completely solve the under-triage problem and that the problem isn’t entirely due to physicians’ diagnostic skills. But it’s heartening to know we’re on track to develop a game that shows promise at improving on current educational training.”
Illustration: Schell Games.
High-Intensity Exercise Delays Parkinson’s Progression

High-intensity exercise three times a week is safe for individuals with early-stage Parkinson’s disease and decreases worsening of motor symptoms. These results are according to a new phase 2, multi-site trial led by Northwestern Medicine and University of Colorado School of Medicine scientists. The randomized clinical trial included 128 participants ages 40 to 80 years old from Northwestern University, Rush University Medical Center, the University of Colorado, and the University of Pittsburgh. McGowan Institute for Regenerative Medicine affiliated faculty member Anthony Delitto, PhD, Professor and Dean of the School of Health and Rehabilitation Sciences at the University of Pittsburgh, was a member of the Pitt team in this study. The paper was published in JAMA Neurology December 11, 2017.
This is the first-time scientists have tested the effects of high-intensity exercise on patients with Parkinson’s disease, the second most common neurodegenerative disorder and the most common movement disorder, affecting more than a million people in the United States.
It previously had been thought high-intensity exercise was too physically stressful for individuals with Parkinson’s disease.
Parkinson’s symptoms include progressive loss of muscle control, trembling, stiffness, slowness, and impaired balance. As the disease progresses, it may become difficult to walk, talk, and complete simple tasks. Most people who develop Parkinson’s are 60 and older.
“If you have Parkinson’s disease and you want to delay the progression of your symptoms, you should exercise three times a week with your heart rate between 80 to 85 percent maximum. It is that simple,” said co-lead author Daniel Corcos, PhD, professor of physical therapy and human movement sciences at Northwestern University Feinberg School of Medicine.
Because medications for Parkinson’s have adverse side effects and reduced effectiveness over time, new treatments are needed.
Participants enrolled in the Study in Parkinson Disease of Exercise (SPARX) were at an early stage of the disease and not taking Parkinson’s medication, ensuring the results of the study were related to the exercise and not affected by medication.
“The earlier in the disease you intervene, the more likely it is you can prevent the progression of the disease,” Dr. Corcos said. “We delayed worsening of symptoms for six months; whether we can prevent progression any longer than six months will require further study.”
Scientists examined the safety and effects of exercise three times weekly for six months at high intensity, 80 to 85 percent of maximum heart rate, and moderate intensity, 60 to 65 percent of maximum heart rate. They compared the results to a control group who did not exercise.
After six months, participants were rated by clinicians on a Parkinson’s disease scale ranging from 0 to 108. The higher the number, the more severe the symptoms.
Participants in the study had a score of about 20 before exercise. Those in the high intensity group stayed at 20. The group with moderate exercise got worse by 1.5 points. The group that did not exercise worsened by three points. Three points out of a score of 20 points is a 15 percent change in the primary signs of the disease and considered clinically important to patients. It makes a difference in their quality of life.
“We are stopping people from getting worse, which is significant, particularly if we catch them early in the disease,” Dr. Corcos said.
What sets this study apart from others is the high number of participants, and that they exercised for a relatively long period of time. Most exercise studies are 12 weeks, Dr. Corcos said.
“We gave them a proper workout,” Dr. Corcos said. “This is not mild stretching. This is high intensity. It’s part of the idea that exercise is medicine.”
Dr. Corcos and colleagues confirmed it was safe for the participants to do high-intensity exercise by giving them a cardiologist-supervised graded exercise test to evaluate the heart’s response to exercise.
Previous studies in humans suggest high-intensity exercise improves motor symptoms, but the evidence wasn’t sufficient to determine whether exercise intensity modifies symptoms or disease progression. In addition, most studies have not precisely measured or controlled exercise intensity and none have been conducted at 80 to 85 percent maximum heart rate.
“Several lines of evidence point to a beneficial effect of exercise in Parkinson’s disease,” said Dr. Codrin Lungu, program director at the National Institute of Neurological Disorders and Stroke. “Nevertheless, it’s not clear which kind of exercise is most effective. The SPARX trial tries to rigorously address this issue. The results are interesting and warrant further exploration of the optimal exercise regimes for Parkinson’s.”
International Consensus Meeting Led to Insight on Ankle Cartilage Treatments

As reported by Casey Tingle, staff writer for Orthopedics Today, ankle cartilage surgeons from around the world collaborated in Pittsburgh at the International Consensus Meeting on Cartilage Repair of the Ankle, where they discussed and debated the best ways to treat ankle cartilage injuries and ultimately established a consensus to which all orthopedic surgeons can refer.
“We definitely have more insight on evidence-based approaches to ankle cartilage injury treatment and it came together with a solid consensus on how these injuries can be treated, at least consistently within the evidence across the globe,” said McGowan Institute for Regenerative Medicine affiliated faculty member MaCalus Hogan, MD, chief of the division of foot and ankle surgery in the department of orthopedic surgery at University of Pittsburgh and local co-host of the meeting.
Dr. Hogan said it was critical, through the meeting, to bring together the resources, knowledge and tools that most surgeons who treat these complex injuries have at their disposal and to develop “a consensus statement within which all surgeons can function reliably and feel confident that they are approaching this in an evidence-based manner.”
The consensus process began 1 year prior to the meeting, which was held November 2017. Participants answered 137 questions related to 12 domains of ankle cartilage repair, including general principles and terminology, diagnosis, treatment methods, rehabilitation and follow-up.
“This culminated in a 140-page document of which 90% was agreed upon prior to the Pittsburgh meeting,” John Kennedy, MD, FRCS, of Hospital for Special Surgery, co-founder of the International Society on Cartilage Repair of the Ankle (ISCRA), said. “The remaining 10% of agreement was reached by consensus” at the meeting, he said.
Along with publishing the consensus, Richard Ferkel, MD, co-founder of ISCRA, said organizers expect to publish separate articles in other arthroscopy and foot and ankle journals on each domain discussed, as well as summaries of the consensus, so more physicians have access to the information.
“Then we are going to have a complete … supplement in a journal that will have the entire consensus meeting with each individual section having its own article to describe the entire process and the recommendations,” said Dr. Ferkel of Southern California Orthopedic Institute. “We are going to work hard to make sure that comes out as soon as we can in 2018, so that it is timely in nature.”
The Brain Is Less Flexible Than Previously Thought When Learning

Nobody really knows how the activity in your brain reorganizes as you learn new tasks, but new research from Carnegie Mellon University and the University of Pittsburgh reveals that the brain has various mechanisms and constraints by which it reorganizes its neural activity when learning over the course of a few hours. McGowan Institute for Regenerative Medicine affiliated faculty member Elizabeth Tyler-Kabara, MD, PhD, associate professor in the Departments of Neurological Surgery, Bioengineering, and Physical Medicine and Rehabilitation at the University of Pittsburgh and the director of the Spasticity and Movement Disorder Program at Children’s Hospital of Pittsburgh of UPMC, is co-author of the new research which finds that, when learning a new task, the brain is less flexible than previously thought.
The research, published recently in Nature Neuroscience (DOI: 10.1038/s41593-018-0095-3), examined the changes that take place in the brain when learning a new task. To truly see how neural activity changes during learning, we need to look bigger—at populations of neurons, rather than one neuron at a time, which has been the standard approach to date.
The research team used a brain-computer interface (BCI), where subjects move a cursor on a computer screen by thought alone. As with learning to play a new sport, they found that subjects learned to control the cursor more accurately with practice. They then investigated how the activity in the brain changed during learning that enabled the improved performance. They found that, on a time scale of a few hours, the brain does not reconfigure its neural activity to maximize the speed and accuracy by which it moves the cursor.
“In this experimental paradigm, we’re able to track all of the neurons that can lead to behavioral improvements and look at how they all change simultaneously,” says Steve Chase, PhD, an associate professor of biomedical engineering at Carnegie Mellon and the Center for the Neural Basis of Cognition. “When we do that, what we see is a really constrained set of changes that happen, and it leads to this suboptimal improvement of performance. And so, that implies that there are limits that constrain how flexible your brain is, at least on these short time scales.”
When we’re learning a new task, we can’t instantaneously learn it to proficiency, in part due to the way in which the neurons are wired up in the brain. Learning takes time, and there are mechanisms by which neurons can change the way they communicate with each other to enable learning—some of which can be fast, and some of which can take longer. The team found that the brain operates under a more stringent set of constraints than originally thought, resulting in good learning on the short term, but nevertheless suboptimal performance in controlling the BCI cursor.
Imagine a tennis player whose friends have asked her to play squash. When she picks up the squash racket, it’s lighter than the tennis racket she is used to, and it has a slightly different balance point. But since she’s a good tennis player, this difference in rackets doesn’t cause her to miss the ball completely. She adjusts quickly, but she hasn’t immediately picked up the swing form of a squash player. To really become an expert, it will require a long period of training with the new equipment. However, her experienced squash-playing friends will quickly see that she is a tennis player, because until she’s learned the proper technique, she’ll be swinging the squash racket the same as she would a tennis racket.
“Just as it takes time to train a person to swing a squash racket like an expert, it takes time to train one’s neurons to produce the ideal activity patterns,” says Byron Yu, PhD, associate professor of biomedical engineering and electrical and computer engineering at Carnegie Mellon. “When faced with a new task, we’re finding that the brain is constrained to take the neural activity patterns that it’s capable of generating right now and use them as effectively as possible in this new task.”
“When we learn, at first the brain tends to not produce new activity patterns, but to repurpose the activity patterns it already knows how to generate,” says Aaron Batista, PhD, an associate professor in the Department of Bioengineering at the University of Pittsburgh. “Learning over the course of a few hours is suboptimal. When first learning something new, our brain doesn’t seem to be able to change its activity in the best possible way to allow us to be proficient at new skills.”
Acquiring a skill is very difficult, and it takes a lot of time and a lot of practice. But when you’re first starting to learn a new skill, your brain has to adjust quickly to the new task. The researchers found that the brain is constrained to take neural activity patterns it already knows and use them for the new task. By repurposing neuron patterns the brain is already capable of generating, the brain applies a “quick and dirty fix” to the new problem it’s facing.
“None of us predicted this outcome,” says Matthew Golub, PhD, a postdoctoral researcher in electrical and computer engineering at Carnegie Mellon. “Learning is far more limited on the scale of a few hours than any of us were expecting when we started this. We were all surprised that the brain wasn’t able to choose the best strategy possible.”
The research was done in collaboration with the Center for Neural Basis of Cognition, a cross-university research and educational program between Carnegie Mellon and the University of Pittsburgh that leverages each institution’s strengths to investigate the cognitive and neural mechanisms that give rise to biological intelligence and behavior.
Scientists Identify Mechanism of Impaired Dendritic Cell Function that Weakens Immune and Therapeutic Response to Cancer

A new study from The Wistar Institute, in collaboration with the University of Pittsburgh and others, revealed the mechanism implicated in the defective function of tumor-associated dendritic cells (DCs), a specialized type of immune cells that expose the antigens on their surface to activate the T cells. The new findings explain why DCs are not effective in executing a specialized process that is required for inducing antitumor immune responses and effective cancer immunotherapy. The work was published online in Nature Communications. McGowan Institute for Regenerative Medicine affiliated faculty member Valerian Kagan, PhD, Professor and Vice-Chairman in the Department of Environmental and Occupational Health at Pitt, is a co-author on the paper.
“Dendritic cells are essential for prompting the immune response against malignant cells and for driving the clinical success of cancer immunotherapy, but their function is often defective in cancer patients,” said Dmitry I. Gabrilovich, MD, PhD, Christopher M. Davis Professor and program leader of the Immunology, Microenvironment & Metastasis Program at Wistar. “Our research sheds light on the mechanism of this impairment, pointing us in the direction of new strategies for improving the response to immunotherapy.”
DCs are specialized immune cells that can ingest foreign antigens, degrade them, and present the fragments on their surface to activate T cell-mediated immunity. Antigens are exposed on the surface of DCs in a protein complex along with major histocompatibility complex (MHC) proteins that mediate the recognition and activation of the appropriate T cell subtype. Different classes of MHC molecules exist and are involved in immunity against pathogens and tumor cells as well as the formation of immune tolerance to self-antigens.
DCs are fundamental players in antitumor immunity through a process known as antigen cross-presentation, in which they process tumor-derived antigens into the MHC class I pathway for presentation to T cells that have the ability to kill cancer cells. This DC function is also required for the success of cancer vaccines and immunotherapy, but it is well established that tumor-associated DCs are defective in their ability to perform antigen cross-presentation. DCs from cancer patients and tumor-bearing animal models also accumulate higher amounts of lipids compared with DCs from healthy individuals, a process that has been implicated in defective cross-presentation by DCs. The mechanism underlying this association was unknown.
In collaboration with the group of Dr. Kagan at Pitt, Dr. Gabrilovich and colleagues analyzed in great detail the events that take place in the DCs from tumor-bearing mice models and found that impaired cross-presentation, which occurred in the presence of tumor-derived factors, was associated with defective trafficking of the antigen-MHC complex to the cell surface. Importantly, they also observed that tumor-associated DCs accumulate chemically altered lipids that are the product of oxidative modification. Compared to unaltered lipids, these modified lipids had different ability to interact with target proteins, thus modifying their function.
Computational analysis as well as direct experiments identified some of the target proteins that strongly interact with the modified lipids, showing that these proteins are involved in the transport of the antigen-MHC complex to the cell surface. Interfering with the function of their targets, these interactions ultimately impair cross-presentation.
“Our findings present new potential targets for therapeutic regulation of cross-presentation,” said Filippo Veglia, PhD, first author of the study and staff scientist in the Gabrilovich Lab. “For example, we investigated the effect of the antioxidant vitamin E and found that it abrogated the tumor-induced defects in cross-presentation by DCs.”
SHRS and Dr. David Brienza Receive $5 Million Grant from National Institute on Disability/U.S. Department of Education

As reported by Liz Beaulieu for HME News, McGowan Institute for Regenerative Medicine affiliated faculty member David Brienza, PhD, professor in the Department of Rehabilitation Science and Technology and associate dean for research in the School of Health and Rehabilitation Sciences (SHRS), and researchers at the University of Pittsburgh have secured a nearly $5 million grant to continue their work developing standards to improve product quality and safety for wheelchairs.
Wheelchair quality and safety are a growing problem, the researchers say, with about 50% of users saying they have experienced a breakdown in a six-month period, according to research they have already conducted.
“This grant allows us to have a real impact,” said Jon Pearlman, PhD, an associate professor in the Department of Rehabilitation Science and Technology at SHRS. “In the research community, you’re often awarded grants for thinking outside the box and for working on products that might be realistic in 10 years. This is more practical.”
The “Rehabilitation Engineering Research Center” grant, awarded by the National Institute on Disability, a federal government agency within the U.S. Department of Education, will provide more than $900,000 each year for five years.
Researchers plan to spend the first few years of the grant improving or adding to existing standards, particularly related to cushion load-bearing performance, cushion durability, caster durability, and wheel rolling resistance, and the remaining few years applying those standards to different products.
“We want to show the differentiations between products and the usefulness of that information,” said Patricia Karg, MSE, an assistant professor in the Department of Rehabilitation Science and Technology at SHRS. “We want to translate standards into strategies and techniques for selecting products based on performance.”
The problem with existing standards, which have been developed over decades by national and international standards committees: they’re largely influenced by product manufacturers, the researchers say.
“The awarding of this grant will allow for some unbiased participation,” said Dr. Brienza. “Manufacturers have a large stake in standards and how they’re developed, and they support the process. This balances that process.”
Ultimately, the researchers envision their work being used in several ways, including helping funding sources make reimbursement decisions, and clinicians, providers, and users make product decisions.
“It’s in this way that standards, which are often voluntary and which until now didn’t differentiate between products, will get teeth,” Dr. Brienza said.
Illustration: SHRS.
Dr. Shawn Kelly Receives Project Funding from the Chuck Noll Foundation

The Chuck Noll Foundation for Brain Injury Research awarded more than $600,000 in grants to five research teams at the University of Pittsburgh, Carnegie Mellon University, and UPMC in its first-year distribution.
Automated detection and suppression of brain tsunamis is the topic of a 3-year study by Pulkit Grover, PhD, Marlene Behrmann, PhD, Michael Tarr, PhD, and Shawn Kelly, PhD, of Carnegie Mellon University, and Jonathan Elmer, MD, and Lori Shutter, MD, of Pitt. The team is looking to develop a concussion monitoring and treatment system. Dr. Kelly is a McGowan Institute for Regenerative Medicine affiliated faculty member.
The foundation was started in 2016 by the Pittsburgh Steelers with an initial contribution of $1 million and the mission to support continued research and education on brain injuries and treatment of sports-related concussions. Mr. Noll coached the Steelers for 23 years and led the team to four Super Bowl victories.
Dr. Bradley Nindl: Military Career Leads to Success in the Lab and Beyond

The military experience of McGowan Institute for Regenerative Medicine affiliated faculty member Bradley Nindl, PhD— as a researcher, active duty member, and new commander of a U.S. Army Reserve unit — has enhanced and broadened his work at the University of Pittsburgh.
Dr. Nindl is the director of the Neuromuscular Research Laboratory, the applied research facility of the Department of Sports Medicine and Nutrition within the School of Health and Rehabilitation Sciences, which also includes the Warrior Human Performance Research Center. It’s a post he’s held since 2015 and a continuation of his 25-plus years’ work in the U.S. Army on human performance optimization and injury prevention. His current research at Pitt not only focuses on the cognitive and physical performance of members of the military, but also reaches into the impact of space travel on astronauts in a NASA-funded study.
Additionally, in August 2017, he assumed command of the U.S. Army Reserve Southeast Medical Area Readiness Support Group in Nashville, Tennessee, traveling several times a year for his duties. The group has 2,000 soldiers and provides support, command, and control for 24 direct reporting units that include medical backfill battalions, medical support units, troop medical clinics, and veterinary and blood detachments throughout the southeastern U.S. Dr. Nindl’s knowledge of the military has benefited the Neuromuscular Research Laboratory, which partners with the U.S. Special Operations Command and Marine Corps to study military elite and their unique performance optimization and injury mitigation needs.
“The work of the lab has always been about optimizing performance and preventing injury, and we will continue to do that,” said Dr. Nindl, who is also a professor in the Department of Sports Medicine and Nutrition. “But now we’re moving forward with exciting research that will impact the conventional military population in addition to the Special Forces.”
Dr. Nindl has been collaborating with Shawn Flanagan, PhD, and Chris Connaboy, PhD, assistant professors in Pitt’s Department of Sports Medicine and Nutrition, to study cognitive resilience in military service members. They will be performing some testing at the U.S. Army Reserve unit in Moon Township, Pennsylvania.
The research is testing novel and established measurements of neurocognitive and physical performance during military tasks including marksmanship, adaptive decision making, and spatial navigation.
The trio is also assisting in the NASA-funded astronaut health study led by the University of Pennsylvania, which is examining space travel’s effects on the human body, including behavioral health and performance, cardiovascular alterations, and radiation effects.
Dr. Nindl’s interest in how to maximize physical performance started early — on the court and on the field.
A high school athlete and self-proclaimed “gym rat,” Dr. Nindl played basketball, baseball, and soccer. He also played basketball while studying biology at Clarkson University.
He went on to receive his Bachelor of Science degree in biology from Clarkson in 1989, a Master of Science in physiology of exercise from Springfield College in 1993, his PhD in physiology from Pennsylvania State University in 1999, and a Master of Strategic Studies from the U.S. Army War College in 2012.
While studying for his master’s at Springfield, he attended a class trip to the Army Research Institute of Environmental Medicine, a laboratory of the U.S. Department of Defense based in Natick, Massachusetts, that studies physiology performance in soldiers.
“It just so happened they were recruiting people to enlist in the Army to be research assistants,” Dr. Nindl said. “I thought that was the ideal opportunity to learn the research process and be mentored by top people in the field.”
After being accepted in 1991, he served as a government scientist focusing on human performance optimization and injury prevention. He also studied biomarkers with a focus on adaptations of the neuromuscular and endocrine systems to exercise and military operational stress.
However, being a researcher didn’t exempt Dr. Nindl from being deployed for active duty to Mosul, Iraq, in 2004-05. While there, Dr.Nindl was the executive officer for a Military Transition Team with the 98th Training Division, a group of about 15 soldiers embedded with an Iraqi army unit.
“Our mission was to train Iraqi soldiers and go on operational missions,” Dr. Nindl said. “One of the first things we did in Mosul was to control the battlespace we were operating in and set up polling places for people to go to vote.”
He was awarded a Bronze Star and the Combat Action Badge.
Dr. Nindl said he credits a supportive faculty and family for his ability to handle his research and his service to his country.
“I couldn’t balance everything I’m doing here without the support of my family and my leadership here,” he said. “It’s always a challenge to balance my civilian, military, and family careers. The key to the balancing act I have been doing since 2002 is that I have an incredibly supportive wife and have always had supportive bosses and leaders who appreciated the importance of military service. My department chair, Kevin Conley, PhD, is a supportive chair, great leader, and allows flexibility to allow me to balance my military service.”
AWARDS AND RECOGNITION
Christopher Mahoney, a 2017 Recipient of the TERMIS-AM Student Scientist Award

Christopher Mahoney, bioengineering PhD candidate, is one of the 2017 recipients of the TERMIS-AM Student Scientist award.
The TERMIS-AM Student Scientist Award is given to students who demonstrate passion and promise in their research, academics, and service. It provides financial assistance to undergraduate and graduate students who are presenting in the annual meeting.
Mr. Mahoney’s research advisor, McGowan Institute for Regenerative Medicine faculty member Kacey Marra, PhD, associate professor of plastic surgery and bioengineering, said, “This award is well-deserved as Chris has consistently excelled in all three areas: research, academics, and service.”
The TERMIS-AM annual conference was held December 3-6, 2017, in Charlotte, NC. Mr. Mahoney presented a poster on “Dual Method Verification of Adipogenesis in Cultures Containing an Adipose Derived Delivery System for Adipose Restoration.”
About Christopher Mahoney
Mr. Mahoney works with Dr. Marra on biomaterials and drug delivery for adipose tissue reconstructive applications.
In 2014, Mr. Mahoney received the Wes Pickard Academic Fellowship, which is awarded to students chosen by their department chair who are in good academic standing. In fall 2014, he became a trainee under the University of Pittsburgh’s CATER NIH NRSA Institutional Predoctoral Training Grant (program director: McGowan Institute faculty member Paul Monga, MD), giving Mr. Mahoney the funding and support to develop as a student researcher. In March 2017, he was awarded the highly competitive NIH NRSA Individual Predoctoral Fellowship from the National Institute of Biomedical Imaging and Bioengineering to further his research and complete his doctoral degree.
Mr. Mahoney participates in several other professional and community service activities. He serves on the University Senate Committee on Equality, Inclusion, and Diversity Advocacy and the Graduate and Professional Student Government Event Planning Committee. He is also former president of the Engineering Diversity Graduate Student Association and an active member of the Big Brothers and Big Sisters organization.
Congratulations, Mr. Mahoney!
Illustration: University of Pittsburgh Swanson School of Engineering.

McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, has been inducted as a fellow for the American Association for the Advancement of Science (AAAS).
Dr. Cooper, a University of Pittsburgh professor, School of Health and Rehabilitation Sciences associate dean for inclusion, and Human Engineering Research Laboratories founding director, was elected a fellow “for distinguished contributions to the field of bioengineering and health and rehabilitation sciences, particularly for applications for people with disabilities.”
AAAS is an international nonprofit organization with the stated goals of promoting cooperation among scientists, defending scientific freedom, encouraging scientific responsibility and supporting scientific education and outreach. It is also the publisher of the well-known scientific journal, Science.
Dr. Cooper joins these McGowan Institute affiliated faculty members who are also AAAS fellows: Joseph Glorioso III, PhD, Chien Ho, PhD, Valerian Kagan, PhD, DSc, George Michalopolous, MD, PhD, and Thomas Rando, MD, PhD,
Congratulations, Dr. Cooper!
Dr. Wagner Presents Service Awards
McGowan Institute Director William Wagner, PhD has recognized the following McGowan Institute employees for their sustained service to the University of Pittsburgh:

Five years: James Harris (L) Bioreactor Lab; Joseph Gannon (R) Fiscal Operations

20 years: Joseph Hanke, Center for Preclinical Studies

20 years: Brian Frankowski, Medical Devices Lab
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#181 –– Dr. Jeffrey Isenberg is an Associate Professor in the Department of Medicine at the University of Pittsburgh. Dr. Isenberg discusses his research on controlling blood flow and tissue survival, particularly the thrombospondin-1-CD47 signaling nexus.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, Josbeno DA, Christiansen CL, Berman BD, Kluger BM, Melanson EL, Jain S, Robichaud JA, Poon C, Corcos DM
Title: Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients with De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial
Summary: IMPORTANCE: Parkinson disease is a progressive neurologic disorder. Limited evidence suggests endurance exercise modifies disease severity, particularly high-intensity exercise.
OBJECTIVES: To examine the feasibility and safety of high-intensity treadmill exercise in patients with de novo Parkinson disease who are not taking medication and whether the effect on motor symptoms warrants a phase 3 trial.
DESIGN, SETTING, AND PARTICIPANTS: The Study in Parkinson Disease of Exercise (SPARX) was a phase 2, multicenter randomized clinical trial with 3 groups and masked assessors. Individuals from outpatient and community-based clinics were enrolled from May 1, 2012, through November 30, 2015, with the primary end point at 6 months. Individuals with idiopathic Parkinson disease (Hoehn and Yahr stages 1 or 2) aged 40 to 80 years within 5 years of diagnosis who were not exercising at moderate intensity greater than 3 times per week and not expected to need dopaminergic medication within 6 months participated in this study. A total of 384 volunteers were screened by telephone; 128 were randomly assigned to 1 of 3 groups (high-intensity exercise, moderate-intensity exercise, or control).
INTERVENTIONS: High-intensity treadmill exercise (4 days per week, 80%-85% maximum heart rate [n = 43]), moderate-intensity treadmill exercise (4 days per week, 60%-65% maximum heart rate [n = 45]), or wait-list control (n = 40) for 6 months.
MAIN OUTCOMES AND MEASURES: Feasibility measures were adherence to prescribed heart rate and exercise frequency of 3 days per week and safety. The clinical outcome was 6-month change in Unified Parkinson’s Disease Rating Scale motor score.
RESULTS: A total of 128 patients were included in the study (mean [SD] age, 64 [9] years; age range, 40-80 years; 73 [57.0%] male; and 108 [84.4%] non-Hispanic white). Exercise rates were 2.8 (95% CI, 2.4-3.2) days per week at 80.2% (95% CI, 78.8%-81.7%) maximum heart rate in the high-intensity group and 3.2 (95% CI, 2.8-3.6; P = .13) days per week at 65.9% (95% CI, 64.2%-67.7%) maximum heart rate in the moderate-intensity group (P < .001). The mean change in Unified Parkinson’s Disease Rating Scale motor score in the high-intensity group was 0.3 (95% CI, -1.7 to 2.3) compared with 3.2 (95% CI, 1.4 to 5.1) in the usual care group (P = .03). The high-intensity group, but not the moderate-intensity group, reached the predefined nonfutility threshold compared with the control group. Anticipated adverse musculoskeletal events were not severe.
CONCLUSIONS AND RELEVANCE: High-intensity treadmill exercise may be feasible and prescribed safely for patients with Parkinson disease. An efficacy trial is warranted to determine whether high-intensity treadmill exercise produces meaningful clinical benefits in de novo Parkinson disease.
Source: JAMA Neurol. 2018 Feb 1;75(2):219-226.
GRANT OF THE MONTH
PI: David Brienza
Title: Rehabilitation Engineering Research Center
Description: Researchers plan to spend the first few years of the grant improving or adding to existing standards, particularly related to cushion load-bearing performance, cushion durability, caster durability, and wheel rolling resistance, and the remaining few years applying those standards to different products. Ultimately, the researchers envision their work being used in several ways, including helping funding sources make reimbursement decisions, and clinicians, providers, and users make product decisions.
Source: National Institute on Disability/U.S. Department of Education
Term: 5 years
Amount: $900,000/year