July 2016 | VOL. 15, NO. 7| www.McGowan.pitt.edu
Regenerative Medicine Improves Strength and Function in Severe Muscle Injuries

Results of a study conducted by researchers at the McGowan Institute for Regenerative Medicine and the University of Pittsburgh School of Medicine showed significant improvement in strength and range of motion, as well as evidence for skeletal muscle regeneration, in 13 patients who were surgically implanted with bioscaffolds derived from pig tissue to treat muscle injuries. The patients had failed to respond to conventional treatment before use of the extracellular matrix (ECM). The findings were published online in npj Regenerative Medicine.
“Previously, there was no effective treatment for these patients, but this approach holds significant promise,” said senior investigator Stephen Badylak, DVM, PhD, MD, professor of surgery at Pitt and deputy director of the McGowan Institute, a joint effort of Pitt and UPMC. “This approach could be a game changer and not just an incremental advance.” Other McGowan Institute for Regenerative Medicine affiliated faculty members participating in the study include: Fabrisia Ambrosio, PhD, Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh, Neill Turner, PhD, Research Assistant Professor in the Department of Surgery at the University of Pittsburgh, Michael Boninger, MD, UPMC’s Vice President for Medical Affairs for Community Provider Services, and J. Peter Rubin, MD, UPMC Professor and Chair of Plastic Surgery, Pitt School of Medicine.
For the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive Surgery Research Study, which was sponsored by the U.S. Department of Defense, 11 men and 2 women who had lost at least 25 percent of leg or arm muscle volume and function first underwent a customized regimen of physical therapy for 4 to 16 weeks.
Lead study surgeon Dr. Rubin then surgically implanted a “quilt” of compressed ECM sheets designed to fill in their injury sites. Within 48 hours of the operation, the participants resumed physical therapy for up to 24 additional weeks.
By 6 months after implantation, patients showed an average improvement of 37.3 percent in strength and 27.1 percent in range of motion tasks compared with pre-operative performance numbers. CT or MRI imaging also showed an increase in post-operative soft tissue formation in all 13 patients.
“For well-selected patients with this type of loss, we now have a treatment available to help improve their function,” Dr. Rubin said.
The new data builds upon a previous Pitt study that showed damaged leg muscles grew stronger and showed signs of regeneration in three out of five men whose old injuries were surgically implanted with ECM derived from pig bladder. Those patients also underwent similar pre- and post-operative physical therapy.
The recent results included more patients with varying limb injuries; used three different types of pig tissues for ECM bioscaffolds; investigated neurogenic cells as a component of the functional remodeling process; and included CT and MRI imaging to evaluate the remodeled muscle tissue. In this video, study participant Maury Noonan explains how the regenerative medicine procedure and physical therapy aided his recovery.
“The three different types of matrix materials used all worked the same, which is significant because it means this is a generic property of these materials and gives the surgeons a choice for using whichever tissue they like,” Dr. Badylak said. “Prior to the surgery, each patient went through physical therapy focused on getting them to the point where they couldn’t get any better. We then started active rehab within 48 hours after surgery, which proved to be critically important for these patients.”
The project was supported by a research grant from the U.S. Department of Defense, grant D11AC00006.
The U.S. Department of Defense’s Limb Salvage and Regenerative Medicine Initiative and the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive Surgery Research Study are collaboratively managed by the Office of the Secretary of Defense. The initiative is focused on rapidly and safely transitioning advanced medical technology in commercially viable capabilities to provide wounded warriors the safest and most advanced care possible today.
Illustration: UPMC YouTube.
Abstract (An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Jenna Dziki, Stephen Badylak, Mohammad Yabroudi, Brian Sicari, Fabrisia Ambrosio, Kristen Stearns, Neill Turner, Aaron Wyse, Michael L Boninger, Elke H. P. Brown and J. Peter Rubin. npj Regenerative Medicine; 1, Article number: 16008 (2016).)
RESOURCES AT THE MCGOWAN INSTITUTE
August Special at the Histology Lab
Safranin O Staining for Cartilage
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
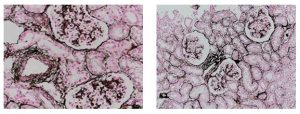 Jones Silver Stain demonstrates basement membrane and reticulin.
Jones Silver Stain demonstrates basement membrane and reticulin.
You’ll receive 25% off Jones Silver Stain in August when you mention this ad. Contact Lori at the McGowan Core Histology Lab by email: perezl@upmc.edu or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
Targeting HIV-1 Nef for New AIDS Therapy Using Humanized Mice – How Flow Cytometry Helps This Project
Efforts at the McGowan Institute Flow Cytometry Core Facility are continuing to successfully support the various research programs of McGowan Institute for Regenerative Medicine and University of Pittsburgh scientists. Flow cytometry is used to perform several procedures including cell counting, cell sorting, detection of biomarkers, and protein engineering. Dr. Sherry Shu is a Research Assistant Professor in the laboratory of Dr. Thomas Smithgall in the University of Pittsburgh’s Department of Microbiology and Molecular Genetics. She shares her experience with the McGowan Institute Flow Cytometry Core Facility personnel here:
Nef is a critical HIV-1 virulence factor that enables infected cells to evade the immune response, avoid superinfection, and produce highly infectious virions. Nef accomplishes these goals by hijacking multiple host pathways involved in cellular activation, protein trafficking, and cell surface receptor expression. We have recently discovered small molecules that bind directly to Nef and disrupt its functions in cells, including Nef-dependent enhancement of infectivity, CD4 and MHC-I downregulation and viral replication. In order to study Nef function and test our compounds in vivo, we are developing humanized models for HIV-1 infection. Humanized mice represent a breakthrough model to study HIV infection and immune responses which previously could only be tested in non-human primates. We generated NSG-BLT (NOD scid gamma mice transplanted with human fetal liver and thymus and injected with human CD34+ stem cells) and hu-PBMC NSG (NOD scid gamma mice injected with human PBMC) mouse models for infection with HIV-1 with wild-type or mutant forms of Nef to test the efficacy of our compounds.
To validate the successful reconstitution and engraftment of human immune cells in these mice, we collaborated with Lynda Guzik at the McGowan Institute Flow Cytometry Core Facility to design a suitable 8-color flow panel to identify all human PBMC subsets with absolute cell counts in mouse blood, and a 7-color panel for detecting HIV-infected human cells in tissues. Figure 1 shows the human immune cell reconstitution (human CD45+ cells versus mouse CD45+ cells) in an NSG-BLT mouse compared to an NSG mouse without engraftment. Human CD4+ and CD8+ T cells as well as the development of B cells can also be observed (Figures 2 and 3). We then used flow to monitor the progression of human CD4+ T cell depletion (model for AIDS) after HIV-1 infection in mice. Ms. Guzik not only runs our experiments on FACSAria II but also provides expertise on analyses, controls, trouble shooting, and reagent choices. We also appreciate that the McGowan Institute Flow Cytometry Core Facility offers various workshops for new technologies related to flow cytometry. We are currently working on detecting HIV-1 infected cells in tissues and hope to evaluate the efficacy of Nef inhibitors in the infected mice soon.
Do not hesitate to contact Ms. Guzik, the Flow Cytometry Facility Manager, for more information on how the Facility’s resources can help you: 412-648-8660 or guzilj@upmc.edu.
SCIENTIFIC ADVANCES
Dr. Rocky Tuan Receives Research Award to Conduct Studies on International Space Station

McGowan Institute for Regenerative Medicine Associate Director Rocky Tuan, PhD, has received a research grant from the Center for the Advancement of Science in Space (CASIS) to continue his work on a 3D microphysiological system (MPS) to be conducted on board the International Space Station (ISS) to evaluate the accelerated aging and degeneration process of bones that occurs in space.
“Studying such rapid progression of the disease offers great advantages to developing treatments for osteoporosis faster and more effectively, in ways that are not possible on Earth,” said Dr. Tuan. “Our research will benefit not only the health of astronauts for long stays in space on the ISS or a future journey to Mars, but also will help people on Earth, providing capabilities for the screening of drug therapies, enhancing personalized medicine, and developing bioreactor technologies for tissue engineering.”
The award is part of the 3D Microphysical Systems for Organs-On-Chips Grand Challenge by CASIS, which was chosen by NASA in 2011 to be the sole manager of the ISS U.S. National Laboratory. This announcement was made at the White House Organ Summit.
Dr. Tuan is director of the Cellular and Molecular Engineering Lab and Distinguished Professor in the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine. He is internationally known for his research in stem cell biology, musculoskeletal tissue engineering, regenerative medicine, and for his innovative leadership role in biomedical education. He also is the director of the Center for Military Medicine Research.
“We are particularly appreciative of the research award from CASIS as recognition of our work on developing veritable models for skeletal tissues that may be used to understand the mechanisms of disease and to expedite drug screening for degenerative conditions such as osteoporosis and osteoarthritis,” Dr. Tuan said.
The award was accepted by Peter Alexander, PhD, and Riccardo Gottardi, PhD, on behalf of Dr. Tuan and the University of Pittsburgh research team.
The MPS project has been partially funded by the Ri.MED Foundation, a collaboration between Italy’s government, the University of Pittsburgh, and UPMC. The foundation, based in Palermo, Italy, promotes, supports, and leads biomedical and biotechnological research projects, with emphasis on the translation of innovative results into clinical practice.
Pitt Researchers Find Key to Parkinson’s Disease Neurodegeneration

Researchers at the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty member Charleen Chu, MD, PhD, Professor in the Department of Pathology, University of Pittsburgh School of Medicine, where she holds the A. Julio Martinez Chair in Neuropathology—have uncovered a major reason why the Parkinson’s-related protein alpha-synuclein, a major constituent of the Lewy bodies that are the pathological hallmark of Parkinson’s disease (PD), is toxic to neurons in the brain. The finding has the potential to lead to new therapies that could slow or stop progression of the devastating illness. The new research appears online in Science Translational Medicine.
PD is a degenerative neurological disease characterized by tremor, slowness, and gait and balance difficulties that affects about 1 million people in the United States. The symptoms are caused by the degeneration and loss of neurons in the brain, particularly those crucial for the initiation and coordination of movement.
“It’s really exciting that we have found a mechanism we can target to create new treatments for this devastating disease,” said lead investigator J. Timothy Greenamyre, MD, PhD, Love Family Professor of Neurology in Pitt’s School of Medicine and director of the Pittsburgh Institute for Neurodegenerative Diseases (PIND).
PIND’s goal is an integrated, interdisciplinary approach to the study of neurodegenerative diseases and their mechanisms, with the aim of transforming cutting-edge science into novel therapies and diagnostics that directly benefit individuals affected by neurodegenerative diseases.
“With four different PIND investigators working together, the new study highlights the power of this collaborative approach,” Dr. Greenamyre added.
Current treatments for PD can reduce symptoms, but they do not slow the inevitable worsening of the disease. To slow or halt illness progression, scientists must first determine why and how the neurons are dying.
Degenerating neurons contain large clumps of a protein called alpha-synuclein. People whose cells make too much alpha-synuclein or make a mutated form of the protein are at high risk of developing PD because of the protein’s toxicity, researchers found. Scientists also demonstrated that the accumulation of alpha-synuclein in PD is toxic because it disrupts the normal functioning of mitochondria—the tiny powerhouses responsible for generating a cell’s energy.
Ultimately, this interaction between alpha-synuclein and TOM20 leads to neurodegeneration, Dr. Greenamyre explained.
The researchers then confirmed their animal findings in brain tissue from people with PD.
“The effects of alpha-synuclein on mitochondria are like making a perfectly good coal-fueled power plant extremely inefficient, so it not only fails to make enough electricity, but also creates too much toxic pollution,” said Dr. Greenamyre.
Using cell cultures, the research team also found two ways to prevent the toxicity caused by alpha-synuclein: gene therapy that forced the neurons to make more TOM20 protein protected them from the alpha-synuclein; and a protein that was able to prevent alpha-synuclein from sticking to TOM20 prevented alpha-synuclein’s harmful effects on mitochondria.
While more research is needed to determine whether these approaches could help PD patients, Dr. Greenamyre is optimistic that one or both may ultimately make it into human clinical trials in an effort to slow or halt the otherwise inevitable progression of PD.
Coauthors of the study are Edward Burton, MD, PhD, Teresa Hastings, PhD, Eric Hoffman, PhD, Caitlyn Barrett, PhD, Alevtina Zharikov, PhD, Anupom Borah, PhD, Xiaoping Hu, BS, and Jennifer McCoy, BS, all of PIND.
Abstract (α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Roberto Di Maio, Paul J. Barrett, Eric K. Hoffman, Caitlyn W. Barrett, Alevtina Zharikov, Anupom Borah, Xiaoping Hu, Jennifer McCoy, Charleen T. Chu, Edward A. Burton4, Teresa G. Hastings and J. Timothy Greenamyre. Science Translational Medicine; 08 Jun 2016, Vol. 8, Issue 342, pp. 342ra78.)
Fabrication of Vascular Grafts with Artificial Stem Cells
The National Institutes of Health has awarded McGowan Institute for Regenerative Medicine faculty member David Vorp, PhD, the William Kepler Whiteford Professor of Bioengineering and Associate Dean for Research of the Swanson School of Engineering at Pitt, and colleagues with a grant worth more than $1.54 million to fund their study investigating artificial stem cells in the development of engineered vascular grafts. Dr. Vorp is joined on this study by McGowan Institute for Regenerative Medicine colleagues Steven Little, PhD, the William Kepler Whiteford Professor and Chair of Chemical Engineering; William Wagner, PhD, Professor of Surgery and Director of the McGowan Institute; and Justin Weinbaum, PhD, Research Assistant Professor of Bioengineering; along with Pitt colleague Morgan Fedorchak, PhD, Assistant Professor of Opthalmology.
Some current regenerative medicine approaches use mesenchymal stem cells (MSCs) harvested from the patient to help rebuild or repair damaged or diseased tissues. Dr. Vorp and his team have pioneered the use of MSCs in the development of tissue-engineered vascular grafts (TEVGs), which may be effective in small diameter arterial bypass procedures or arteriovenous access for dialysis. However, MSCs taken from patients at high risk for cardiovascular disease, such as the elderly and diabetics, may be dysfunctional. Furthermore, the use of harvested cells that require extended culture expansion also runs the risk of cellular contamination or transformation, as well as high costs and substantial waiting time before a graft can be made and implanted.
“Fully functional human MSCs secrete a host of biochemicals, including those that prevent blood clotting and those that ‘call’ into the TEVGs important cells from the host, such as inflammatory cells, smooth muscle cells, and endothelial cells,” said Dr. Vorp. “We have found that MSCs from diabetics, for example, are relatively ineffective in yielding a successful TEVG compared to MSCs from non-diabetics. Considering that diabetics make up a large proportion of patients who need bypass grafts, we needed to find an alternative means to achieve our goal for this significant population.”
To answer this challenge, the research team has developed artificial stem cells (artMSCs) that are created by encapsulating the veritable cocktail of biochemicals secreted by normally functioning MSCs in culture into biodegradable microspheres that are similar in size to actual MSCs.
“By ‘tuning’ or adjusting the degradation rate of the microspheres, we can replicate the release of these biochemicals by real, fully-functional MSCs,” said Dr. Vorp. He and his colleagues will then seed the artMSCs into porous, tubular scaffolds and implant them in a rat model as they have done with MSCs in fabricating TEVGs.
The study, entitled “Artificial Stem Cells for Vascular Tissue Engineering,” aims to accelerate the clinical translation of the team’s TEVG technology. This will be achieved, according to Dr. Vorp, “both by making the technology applicable to all patients – even those with dysfunctional MSCs – and by reducing the regulatory barriers associated with the need for culture-expanding cells to the numbers necessary to fabricate a TEVG.”
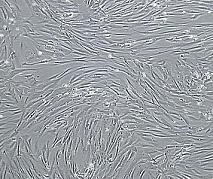
Illustration: Vascular smooth muscle cells, isolated from human aorta, growing and forming a monolayer in cell culture. Wikipedia.
Drs. Robert Parker and Gilles Clermont Receive NIH R21 Award
 The National Heart, Lung, and Blood Institute of the National Institutes of Health announced the Exploratory/Developmental Research Grant Award (R21) to McGowan Institute for Regenerative Medicine affiliated faculty members Robert Parker, PhD, Professor in the Department of Chemical and Petroleum Engineering at the Swanson School of Engineering at the University of Pittsburgh, a Member of the Molec
The National Heart, Lung, and Blood Institute of the National Institutes of Health announced the Exploratory/Developmental Research Grant Award (R21) to McGowan Institute for Regenerative Medicine affiliated faculty members Robert Parker, PhD, Professor in the Department of Chemical and Petroleum Engineering at the Swanson School of Engineering at the University of Pittsburgh, a Member of the Molec
ular Therapeutics/Drug Discovery Program at the University of Pittsburgh Cancer Institute, and a Member of the Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center in the Department of Critical Care Medicine at the University of Pittsburgh, and Gilles Clermont, MD, Professor of Critical Care Medicine, of Industrial Engineering, and of Mathematics at the University of Pittsburgh, and the Medical Director of the Center for Inflammation and Regenerative Modeling, University of Pittsburgh, for their proposal titled “Endotypes of Thrombocytopenia in the Critically Ill.” This award is for $275,000 over the next 2 years.
Congratulations, Drs. Parker and Clermont!
Stanford and Pitt Collaborate on Successful Stroke Therapy
Injecting modified, human, adult stem cells directly into the brains of chronic stroke patients proved not only safe but effective in restoring motor function, according to the findings of a small clinical trial led by Stanford University School of Medicine investigators. Approximately 1/3 of the patients received their treatment at the University of Pittsburgh Medical Center (UPMC). The Pitt portion of the study was under the leadership of Lawrence Wechsler, MD, Henry B. Higman Professor and Chair, Department of Neurology, and Vice President for Telemedicine, UPMC.
The patients, all of whom had suffered their first and only stroke between 6 months and 3 years before receiving the injections, remained conscious under light anesthesia throughout the procedure, which involved drilling a small hole through their skulls. The next day they all went home.
Although more than 3/4 of them suffered from transient headaches afterward — probably due to the surgical procedure and the physical constraints employed to ensure its precision — there were no side effects attributable to the stem cells themselves, and no life-threatening adverse effects linked to the procedure used to administer them, according to a paper, published online in Stroke, that details the trial’s results.
The promising results set the stage for an expanded trial of the procedure now getting underway. They also call for new thinking regarding the permanence of brain damage, said Gary Steinberg, MD, PhD, professor and chair of neurosurgery, Stanford. Dr. Steinberg, who has more than 15 years’ worth of experience in work with stem cell therapies for neurological indications, is the paper’s lead and senior author.
“This was just a single trial, and a small one,” cautioned Dr. Steinberg, who led the 18-patient trial and conducted 12 of the procedures himself. (The rest were performed at the University of Pittsburgh Medical Center.) “It was designed primarily to test the procedure’s safety. But patients improved by several standard measures, and their improvement was not only statistically significant, but clinically meaningful. Their ability to move around has recovered visibly. That’s unprecedented. At 6 months out from a stroke, you don’t expect to see any further recovery.”
Some 800,000 people suffer a stroke each year in the United States alone. About 85 percent of all strokes are ischemic: They occur when a clot forms in a blood vessel supplying blood to part of the brain, with subsequent intensive damage to the affected area. The specific loss of function incurred depends on exactly where within the brain the stroke occurs, and on its magnitude.
Although approved therapies for ischemic stroke exist, to be effective they must be applied within a few hours of the event — a time frame that often is exceeded by the amount of time it takes for a stroke patient to arrive at a treatment center.
Illustration: Clipart.
AWARDS AND RECOGNITION
2016 Beckman Scholar: To Further Her Research in Tissue Engineering Innovations

University of Pittsburgh’s 2016 Beckman Scholar—senior Uma Balakrishnan—is working toward a medical career and seeks to make significant contributions to research in human tissue engineering. Ms. Balakrishnan’s research focuses on finding new methods for producing high-cell density tissues in laboratory settings. Working within the lab of McGowan Institute for Regenerative Medicine affiliated faculty member Lance Davidson, PhD, Associate Professor and Wellington C. Carl Faculty Fellow in the Department of Bioengineering, she is looking for alternative methods of tissue generation, which could have an impact in organ repairs and transplantation.
“Genetic and tissue engineering have made huge technological leaps over the past decade. We have enormous potential to affect human development, and I see tissue engineering as the next frontier for medical advancement,” said Ms. Balakrishnan, a bioengineering major within the Swanson School of Engineering. “I look to one day be at the cutting edge of researching and implementing relevant clinical applications for tissue engineering, playing an active role in the development of natural biological mechanisms for disease prevention and treatment.”
A native of Iowa City, Iowa, Ms. Balakrishnan, a Pitt Chancellor’s Scholar, founded the University of Pittsburgh Rotaract Club, a program of Rotary International. She serves as a University Honors College Ambassador and an executive board member of the Theta Tau professional engineering fraternity. She also has studied abroad at the University of Alcalá in Spain and the University of Economics and Finance in Vietnam. Ms. Balakrishnan would like to become a clinician scientist at a major academic medical center.
A 3-year institutional grant, the Beckman Scholars Program Award supports large-scale endeavors of student researchers selected by the awarded institution. It is widely considered one of the nation’s foremost scholarships for preparing undergraduates for careers in the life sciences. The Beckman Scholars Program Award was granted to Pitt in the spring of 2015.
The scholarship provides as much as $26,000 in funding for 16 months of research, consisting of two consecutive summer semesters as well as the intermediate academic year. Scholars present their work to Pitt’s Beckman Scholars Steering and Selection Committee as well as at national conferences and in scholarly journals. Pitt’s University Honors College oversees the University’s Beckman Scholars Program.
“Arnold O. Beckman was an accomplished scientist, and his wife, Mabel M. Beckman, was a world-renowned philanthropist…,” said University Honors College Dean Edward M. Stricker, who serves as director of Pitt’s Beckman Scholars Program. “Through the Beckman Scholars Program Award, the University of Pittsburgh continues to support its community of undergraduate researchers with unique opportunities that pave the way for careers in scientific advancement and innovation.”
Established in 1977, the Arnold and Mabel Beckman Foundation is an independent, nonprofit organization that promotes research in the life-science fields.
Illustration: University of Pittsburgh.
Dr. Freddie Fu Inducted into American Orthopaedic Society for Sports Medicine Hall of Fame

McGowan Institute for Regenerative Medicine affiliated faculty member Freddie Fu, MD was inducted into the American Orthopaedic Society for Sports Medicine’s (AOSSM) Hall of Fame during the Society’s Annual Meeting in Colorado Springs, Colorado. AOSSM Hall of Famers are individuals who have made a substantial contribution to the sports medicine field.
Dr. Fu is a Past AOSSM President and the David Silver Professor and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine. He specializes in sports medicine and holds secondary appointments as Professor of Physical Therapy, Health and Physical Activity, and Mechanical Engineering and serves as the Head Team Physician for the University of Pittsburgh Athletic Department. In 1999, he was awarded an honorary Doctor of Science degree from Point Park University, an honorary Doctor of Public Service degree from Chatham University, and in 2010 was appointed Distinguished Service Professor by the University of Pittsburgh. Dr. Fu received the 2014 Kappa Delta Elizabeth Winston Lanier Award for “Anatomic ACL Reconstruction: A Changing Paradigm” presented by the Kappa Delta Sorority and the Orthopaedic Research and Education Foundation (OREF) and received it at the 2014 Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS).
Dr. Fu’s major research interest lies in anatomic ACL reconstruction, clinical outcomes, and bioengineering of sports-related problems. Dr. Fu has been honored with more than 260 professional awards and honors, made over 1,150 national and international presentations, co-authored 173 books chapters, is an author of over 570 peer-reviewed articles, and edited 30 major orthopaedic textbooks. He is a member and has held offices in numerous academic organizations, including the Herodicus Society and the American Orthopaedic Association. He has served as President of the Pennsylvania Orthopaedic Society, AOSSM and the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS). He has held board positions with the Arthroscopy Association of North America and the Orthopaedic Research and Education Foundation and AOSSM.
Illustration: McGowan Institute for Regenerative Medicine.
Drs. Rory Cooper and Dan Ding Receive Pitt Chancellor’s Innovation Commercialization Funds

The University of Pittsburgh has dedicated $1 million in gap funding over the next 2 years to assist Pitt innovators seeking to commercialize their research discoveries. Coordinated through the University’s Innovation Institute, the Chancellor’s Innovation Commercialization Funds will assist faculty and students with Pitt discoveries in identifying unmet needs in the market for their innovations, developing prototypes, identifying potential commercial partners, or forming a new enterprise.
The Chancellor’s Innovation Commercialization Funds will be distributed in the following ways:
- Pitt Ventures
- Accelerated Licensing
- Requests for Proposals
- Collaborative Innovation Grants
Two McGowan Institute for Regenerative Medicine affiliated faculty members received funding through the Pitt Ventures program. Pitt Ventures is the Innovation Institute’s program assisting Pitt innovators in moving their innovations through the multiple stages of development toward commercialization. The new funding will provide $400,000 in additional support for this initiative over the next 2 years targeted at innovators participating in the Pitt Ventures Gear Commercialization Program. This includes the upcoming Michael G. Wells Student Healthcare Entrepreneurship Competition and the Kuzneski Innovation Cup, which is focused on non-health care innovations.
The first Chancellor’s Innovation Commercialization Funds have been deployed to four teams that participated in Pitt Ventures’ latest “1st Gear” stage in April. The two winning McGowan Institute affiliated teams are:
Manual Wheelchair Virtual Seating Coach (MVSC)
Innovators: Rory Cooper, PhD, S. Andrea Sundaram: Human Engineering Research Laboratories (HERL)
Description: Pressure sores result from individuals sitting in wheelchairs and not performing frequent-enough weight shifts due to reduced or absent nerve sensation. The resulting ulcers are a significant health concern with negative effects on quality of life and expensive treatments. While the HERL team previously developed a tool to assist those in motorized wheelchairs, until now a solution for those in manual wheelchairs was not available.VIP Wheelchair
Innovators: Dan Ding, PhD, Hyun Ka, PhD: HERL
Description: The VIP Wheelchair is a control system to improve power wheelchair driving accessibility, independence, and safety for people with vision and mobility impairment. The wheelchair incorporates a number of feedback and control mechanisms to help visually impaired users avoid obstacles and drop-offs.
“The spirit of innovation and entrepreneurship is flourishing at Pitt. We are excited to provide additional resources to our faculty and student innovators, helping them translate their innovations into products and services that positively impact society,” said Pitt Chancellor Patrick Gallagher.
“More faculty and students are engaging with the Innovation Institute every year. Through the Chancellor’s Innovation Commercialization Funds, we will be able to meet the rising demand from our Pitt innovators and help them speed the path to market for their discoveries,” said Innovation Institute Founding Director Marc Malandro.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#161 –– Dr. Tarek Fahmy is an Associate Professor of Biomedical Engineering and Immunobiology at Yale University. Dr. Fahmy discusses his research in biomaterials for drug and antigen delivery to the immune system.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
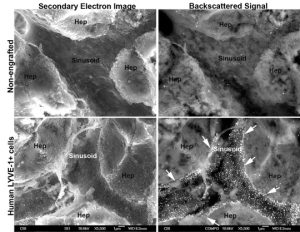
Immuno-SEM evaluation of humans specific LYVE-1 + sinusoids in repopulated mouse liver at low magnification. Bottom panels show a set of human LYVE-1+ cells as determined by strong backscattered signal within the sinusoid (white puncta representing 20 nm gold-conjugated goat anti-rabbit LYVE-1, within the arrows) Top panels show no backscattered signal indicating there is no human LYVE-1+ cells in this sinusoid. Hepatocytes (Hep) also do not stain for LYVE-1.
El Filali, EE, J Hiralall, HA van Veen, DB Stolz, J Seppen. Human liver endothelial cells, but not macrovascular or microvascular endothelial cells engraft in the mouse liver. Cell Transplant. 22(10):1801-1811. 2012. PMID: 23044355.
PUBLICATION OF THE MONTH
Author: Dziki J, Badylak SF, Yabroudi M, Sicari B, Ambrosio F, Stearns K, Turner N, Wyse A, Boninger ML, Brown EHP, Rubin JP
Title: An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study
Summary: Volumetric muscle loss (VML) is a severe and debilitating clinical problem. Current standard of care includes physical therapy or orthotics, which do not correct underlying strength deficits, and surgical tendon transfers or muscle transfers, which involve donor site morbidity and fall short of restoring function. The results of a 13-patient cohort study are described herein and involve a regenerative medicine approach for VML treatment. Acellular bioscaffolds composed of mammalian extracellular matrix (ECM) were implanted and combined with aggressive and early physical therapy following treatment. Immunolabeling of ultrasound-guided biopsies, and magnetic resonance imaging and computed tomography imaging were performed to analyse the presence of stem/progenitor cells and formation of new skeletal muscle. Force production, range-of-motion and functional task performance were analysed by physical therapists. Electrodiagnostic evaluation was used to analyse presence of innervated skeletal muscle. This study is registered with ClinicalTrials.gov, numbers NCT01292876. In vivo remodelling of ECM bioscaffolds was associated with mobilisation of perivascular stem cells; formation of new, vascularised, innervated islands of skeletal muscle within the implantation site; increased force production; and improved functional task performance when compared with pre-operative performance. Compared with pre-operative performance, by 6 months after ECM implantation, patients showed an average improvement of 37.3% (P<0.05) in strength and 27.1% improvement in range-of-motion tasks (P<0.05). Implantation of acellular bioscaffolds derived from ECM can improve strength and function, and promotes site-appropriate remodelling of VML defects. These findings provide early evidence of bioscaffolding as a viable treatment of VML.
Source: npj Regenerative Medicine. 21 July 2016.
GRANT OF THE MONTH
PI: Robert Parker and Gilles Clermont
Title: Endotypes of thrombocytopenia in the critically ill
Description: Thrombocytopenia is extremely frequent in critically ill patients. However, the role of acute platelet responses in critically ill patients is not well studied, and the multifactorial etiology of thrombocytopenia in the ICU makes it difficult to understand, or understand whether or not to treat it. In several situations such as traumatic injury or sepsis, very low platelet counts have been to bleeding, thrombosis and end-organ injury. Platelets have been extensively studied as a key component of hemostasis, but a rapidly emerging concept is that platelets are also key effector cells in systemic inflammatory processes as both instigators of local and systemic inflammatory reactions and also participants in the inflammation that contributes to tissue injury. The link between platelets and inflammation is complex and bidirectional, as inflammatory ligands have been shown to regulate platelet function and activated platelets induce inflammatory responses in other cell types. The overarching theme of this proposal is to study platelet dynamics in critically ill patients, construct clinical endotypes of thrombocytopenia in this population, and to relate these endotypes to underlying mesoscale mechanisms through computational modeling. We will use a large electronic health record-based database and a tri-state trauma database as source data to construct these endotypes. We define endotype as clinical patterns defined along four dimensions: (1) baseline information (demographic, chronic disease burden, severity of illness and admitting diagnosis), (2) features of the platelet count time series (rate of decrease, nadir, etc.), (3) concurrent interventions, and (4) outcome. The computational approach will attempt to root clinical endotypes in mechanistic interpretations (or collections of alternative interpretations), contributing to focus basic science investiagtions, and to close key knowledge gaps preventing the design and use of targeted anti-platelet-inflammatory therapies in the critically ill. Computational models will be developed at different levels of complexity, with a specific attention to tie underlying mechanisms to functional assays routinely performed in thrombocytopenic patients, such as prothrombin time, activated coagulation time, and thromboelastogram.
Source: National Heart, Lung, & Blood Institute
Term: 2 years
Source: $217,444
