January 2021 | VOL. 20, NO. 1| www.McGowan.pitt.edu
Breathing Easier with a Better Tracheal Stent

Pediatric laryngotracheal stenosis (LTS), a narrowing of the airway in children, is a complex medical condition. While it can be something a child is born with or caused by injury, the condition can result in a life-threatening emergency if untreated. Treatment, however, is challenging. Depending on the severity, doctors will use a combination of endoscopic techniques, surgical repair, tracheostomy, or deployment of stents to hold the airway open and enable breathing.
While stents are great at holding the airway open and simultaneously allowing the trachea to continue growing, they can move around, or cause damage when they’re eventually removed. New research published in Communications Biology and led by the University of Pittsburgh is poised to drastically improve the use of stents, demonstrating for the first time the successful use of a completely biodegradable magnesium-alloy tracheal stent that avoids some of these risks. McGowan Institute for Regenerative Medicine affiliated faculty members who are co-authors on the study include:
- Youngjae Chun, PhD, Associate Professor, Department of Industrial Engineering, with a secondary appointment in Department of Bioengineering at the University of Pittsburgh
- William Wagner, PhD, Director of the McGowan Institute as well as a Distinguished Professor of Surgery, Bioengineering and Chemical Engineering at the University of Pittsburgh
- Prashant Kumta, PhD, Edward R. Weidlein Chair Professor at the University of Pittsburgh Swanson School of Engineering and School of Dental Medicine, Professor in the Departments of Bioengineering, Chemical and Petroleum Engineering, Mechanical Engineering and Materials Science, and Oral Biology, the Engineering Director of the Center for Craniofacial Regeneration (CCR), and the Founding Director of the Center for Complex Engineered Multi-functional Materials (CCEMM)
- Catalin Toma, MD, Fellow in Interventional Cardiology at the University of Pittsburgh Medical Center
“Using commercial non-biodegradable metal or silicone based tracheal stents has a risk of severe complications and doesn’t achieve optimal clinical outcomes, even in adults,” said Dr. Kumta. “Using advanced biomaterials could offer a less invasive, and more successful, treatment option.”
In the study, the balloon-expandable ultra-high ductility (UHD) biodegradable magnesium stent was shown to perform better than current metallic non-biodegradable stents in use in both in-lab testing and in rabbit models. The stent was shown to keep the airway open over time and have low degradation rates, displaying normal healing and no adverse problems.
“Our results are very promising for the use of this novel biodegradable, high ductility metal stent, particularly for pediatric patients,” said Dr. Kumta. “We hope this new approach leads to new and improved treatments for patients with this complex condition as well as other tracheal obstruction conditions including tracheal cancer.”
The paper, “In-vivo efficacy of biodegradable ultrahigh ductility Mg-Li-Zn alloy tracheal stents for pediatric airway obstruction,” was also authored by the Swanson School’s Jingyao Wu, Abhijit Roy, and Bouen Lee; UPMC’s Leila Mady, Ali Mübin Aral, Humberto E. Trejo Bittar, and David Chi; and Feng Zheng and Ke Yang from The Institute of Metal Research at the Chinese Academy of Sciences.
RESOURCES AT THE MCGOWAN INSTITUTE
February Histology Special
The Histology Core wants to be your Valentine this month with big saving that will warm your heart.
Bring your cardiac tissue to the McGowan Histology Core and receive 25% off your entire order in the month of February when you mention this ad.
You’ll receive 25% off cardiac samples in February when you mention this ad. Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SAVE THE DATE
 20th Annual McGowan Institute Virtual Scientific Retreat
20th Annual McGowan Institute Virtual Scientific Retreat
Registration Now Open for the 2021 McGowan Institute Scientific Retreat
The 20th Annual McGowan Institute for Regenerative Medicine Virtual Scientific Retreat will take place on March 9-11, 2021.
The poster session will begin on the evening of March 9th.
Under the leadership of McGowan Institute for Regenerative Medicine faculty member Bryan Brown, PhD, associate professor, Department of Bioengineering the program committee is planning an exciting group of speakers and topics.
Mark the dates on your calendars. Additional 2021 Retreat details and news will be available here and emailed to registrants.
SCIENTIFIC ADVANCES
Improving the Efficiency of Neural Stem Cell Delivery to the Area of Stroke

Work that was funded through the National Institute of Biomedical Imaging and Bioengineering as part of the Quantum Program and conducted by McGowan Institute for Regenerative Medicine affiliated faculty member Michel Modo, PhD, Professor in the Departments of Radiology and Bioengineering at the University of Pittsburgh, and colleagues, recently was published in the journal Brain Research Bulletin. Dr. Modo is the corresponding author on the study and McGowan Institute deputy director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery at Pitt and Director of the Center for Pre-Clinical Tissue Engineering within the Institute, is a co-author.
In the paper, the researchers report the implantation of “tissue constructs” consisting of neural stem cells and endothelial cells encapsulated in polyethylene glycol microspheres, suspended in extracellular matrix hydrogel into stroke cavities. This dramatically improved the efficiency of neural stem cell delivery to the area of stroke compared to cell suspensions. Conceptually this is a major step forward to potentially build off-the-shelf tissue constructs for the rapid reconstruction of brain tissue, potentially even allow pre-differentiation of these constructs prior to delivery. The manuscript is focused on technical feasibility.
The abstract follows:
Intracerebral implantation of neural stem cells (NSCs) to treat stroke remains an inefficient process with <5% of injected cells being retained. To improve the retention and distribution of NSCs after a stroke, we investigated the utility of NSCs’ encapsulation in polyethylene glycol (PEG) microspheres. We first characterized the impact of the physical properties of different syringes and needles, as well as ejection speed, upon delivery of microspheres to the stroke injured rat brain. A 20 G needle size at a 10 µL/min flow rate achieved the most efficient microsphere ejection. Secondly, we optimized the delivery vehicles for in vivo implantation of PEG microspheres. The suspension of microspheres in extracellular matrix (ECM) hydrogel showed superior retention and distribution in a cortical stroke caused by photothrombosis, as well as in a striatal and cortical cavity ensuing middle cerebral artery occlusion (MCAo). Thirdly, NSCs or NSCs + endothelial cells (ECs) encapsulated into biodegradable microspheres were implanted into a large stroke cavity. Cells in microspheres exhibited a high viability, survived freezing and transport. Implantation of 110 cells/microsphere suspended in ECM hydrogel produced a highly efficient delivery that resulted in the widespread distribution of NSCs in the tissue cavity and damaged peri-infarct tissues. Co-delivery of ECs enhanced the in vivo survival and distribution of ∼1.1 million NSCs. The delivery of NSCs and ECs can be dramatically improved using microsphere encapsulation combined with suspension in ECM hydrogel. These biomaterial innovations are essential to advance clinical efforts to improve the treatment of stroke using intracerebral cell therapy.
Brain Imaging Predicts PTSD After Brain Injury

Post-traumatic stress disorder (PTSD) is a complex psychiatric disorder brought on by physical and/or psychological trauma. How its symptoms, including anxiety, depression and cognitive disturbances arise remains incompletely understood and unpredictable. Treatments and outcomes could potentially be improved if doctors could better predict who would develop PTSD. Now, researchers using magnetic resonance imaging (MRI) have found potential brain biomarkers of PTSD in people with traumatic brain injury (TBI).
The paper, “Smaller Regional Brain Volumes Predict Post-Traumatic Stress Disorder at 3 Months after Mild Traumatic Brain Injury,” was recently published in Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, Professor of Neurological Surgery and Director of the Neurotrauma Clinical Trials Center, Director of Neurotrauma and of the Scoliosis and Spinal Deformity Program at UPMC, and the Clinical Director of the Brain Trauma Research Center, is a co-author of this work.
“The relationship between TBI and PTSD has garnered increased attention in recent years as studies have shown considerable overlap in risk factors and symptoms,” said lead author Murray Stein, MD, MPH, FRCPC, a Distinguished Professor of Psychiatry and Family Medicine & Public Health at the University of California San Diego. “In this study, we were able to use data from TRACK-TBI, a large longitudinal study of patients who present in the Emergency Department with TBIs serious enough to warrant CT (computed tomography) scans.”
The researchers followed over 400 such TBI patients, assessing them for PTSD at 3 and 6 months after their brain injury. At 3 months, 77 participants, or 18 percent, had likely PTSD; at 6 months, 70 participants or 16 percent did. All subjects underwent brain imaging after injury.
“MRI studies conducted within two weeks of injury were used to measure volumes of key structures in the brain thought to be involved in PTSD,” said Dr. Stein. “We found that the volume of several of these structures were predictive of PTSD 3-months post-injury.”
Specifically, smaller volume in brain regions called the cingulate cortex, the superior frontal cortex, and the insula predicted PTSD at 3 months. The regions are associated with arousal, attention, and emotional regulation. The structural imaging did not predict PTSD at 6 months.
The findings are in line with previous studies showing smaller volume in several of these brain regions in people with PTSD and studies suggesting that the reduced cortical volume may be a risk factor for developing PTSD. Together, the findings suggest that a “brain reserve,” or higher cortical volumes, may provide some resilience against PTSD.
Although the biomarker of brain volume differences is not yet robust enough to provide clinical guidance, Dr. Stein said, “it does pave the way for future studies to look even more closely at how these brain regions may contribute to (or protect against) mental health problems such as PTSD.”
U.S. FDA and University of Pittsburgh Announce Collaboration to Research and Develop Innovative Therapies to Help Restore Vision

The University of Pittsburgh announced a collaboration with the U.S. Food and Drug Administration’s Center for Devices and Radiological Health to help address the needs of the visually impaired through the expertise, facilities, and research of the world-class University of Pittsburgh School of Medicine’s Department of Ophthalmology.
Under the agreement, Pitt and the FDA will work together for the next five years on scientific collaborations, educational initiatives and outreach activities designed to address the epidemic of vision loss. As the world’s population continues to grow and age, the number of individuals with visual impairments is expected to triple by the year 2050.
“It’s really exciting to see this happening. This will put Pitt in a position where we can work with the FDA on the validation of new technological approaches by developing programs and protocols,” said José-Alain Sahel, MD, Pitt’s Eye and Ear Foundation Endowed Chair of the Department of Ophthalmology, an affiliated faculty member of the McGowan Institute for Regenerative Medicine, and one of the world’s top experts in retinal diseases and vision restoration research. “There is strong expertise at Pitt that is being recognized at an international level. Patients’ voices will nurture our projects and define the successes we all want to deliver.”
“We appreciate this phenomenal opportunity to partner with the University of Pittsburgh. Developing new methods to assess visual impairment and the impact on daily activities is important to helping the FDA better characterize the consequences of vision loss, and also helping FDA to reliably assess the benefit of novel therapies and rehabilitation technologies,” said Malvina Eydelman, MD, director of the Office of Ophthalmic, Anesthesia, Respiratory, ENT and Dental Devices in the FDA’s Center for Devices and Radiological Health.
Research, facilities, expertise
This agreement allows collaboration on a variety of programs covering shared interests in ophthalmology including collaborative research, public outreach, extension activities, cooperative international initiatives, disciplinary training and exchange of scientists and staff, including sabbaticals, postdoctoral fellowships, and student internships.
It forms the basis for development of scientific collaborations, outreach and educational initiatives between the FDA and the University of Pittsburgh.
Dr. Sahel said Pitt’s expertise combined with the FDA’s regulatory and scientific expertise is intended to allow for research, such as augmented reality headsets or brain stimulation to help people with low vision “see” their surrounding environments better, can be made available sooner.
Pitt’s Department of Ophthalmology is currently developing numerous innovative programs to restore vision or address the needs of the visually impaired: cell therapies, gene therapies, optogenetics, advanced high-resolution imaging, optic nerve regeneration, prosthetic vision, and brain stimulation to restore sight, to name a few. Partnerships with other departments such as the Institute of Rehabilitation at UPMC and the Department of Occupational Therapy at the School of Health and Rehabilitation Sciences provide a strong environment enabling a holistic approach to patients’ needs.
The UPMC Vision and Rehabilitation Tower at UPMC Mercy, expected to be completed in 2022, will provide advanced specialty clinical care and innovative programs for visually impaired patients. Based in Pittsburgh, it also will be the home for the vision research program at Pitt and UPMC.
As founder and director of the Vision Institute in Paris, Dr. Sahel created StreetLab, a not-for-profit naturalistic platform focused on developing and evaluating new products to help people with visual impairments. His team of experts is focused on all the fields where autonomy and accessibility for visually impaired people require improvement: housing, mobility, access to services and work environment, as well as the assessment of the impact innovative therapies and technologies in daily activities. Dr. Sahel is Pitt’s liaison officer in this agreement.
Illustration: The University of Pittsburgh will collaborate with the U.S. Food and Drug Administration’s Center for Devices and Radiological Health to address the needs of the visually impaired through the expertise, facilities, and research of the Pitt School of Medicine’s Department of Ophthalmology. Of the partnership, José-Alain Sahel, Pitt’s Eye and Ear Foundation Endowed Chair of the Department of Ophthalmology, said, “There is strong expertise at Pitt that is being recognized at an international level.” (Tim Betler/UPMC)
Six “Pills” for a Longer and Better Life
Yoram Vodovotz, PhD, Professor of Surgery, University of Pittsburgh, and faculty member of the McGowan Institute for Regenerative Medicine, and Michael Parkinson, MD, MPH, Senior Medical Director of Health and Productivity, UPMC Health Plan & Workpartners, University of Pittsburgh, recently published a piece in The Conversation related to their work issued in the scientific journal Frontiers in Medicine. The latter journal article is the recommendations from the Lifestyle Medicine Research Summit held in Pittsburgh which had the goal to review current status and define research priorities in the six core areas of lifestyle medicine: plant-predominant nutrition, physical activity, sleep, stress, addictive behaviors, and positive psychology/social connection. The Conversation piece is republished below.
The majority of Americans are stressed, sleep-deprived, and overweight and suffer from largely preventable lifestyle diseases such as heart disease, cancer, stroke, and diabetes. Being overweight or obese contributes to the 50% of adults who suffer high blood pressure, 10% with diabetes, and an additional 35% with pre-diabetes. And the costs are unaffordable and growing. About 90% of the nearly $4 trillion Americans spend annually for health care in the U.S. is for chronic diseases and mental health conditions. But there are new lifestyle “medicines” that are free that doctors could be prescribing for all their patients.
Lifestyle medicine is the clinical application of healthy behaviors to prevent, treat, and reverse disease. More than ever, research underscores that the “pills” today’s physician should be prescribing for patients are the six domains of lifestyle medicine: whole food plant-based eating, regular physical activity, restorative sleep, stress management, addiction reduction or elimination, and positive psychology and social connection.
We are a primary care preventive medicine physician (Dr. Parkinson) and a computational immunologist (Dr. Vodovotz), both committed to applying state-of-the-art research to inform the clinical practice of lifestyle medicine. Our findings and recommendations were just published. We highlight the key take-home points for each of the areas below.
Whole-food, plant-based eating
Diets high in fruits, vegetables, and whole grains and lower in animal products and highly processed foods have been associated with prevention of many diseases. These diets have also improved health and even reversed common cardiovascular, metabolic, brain, hormonal, kidney, and autoimmune diseases as well as 35% of all cancers.
We believe that future research should include larger trials or new research methods with emphasis on quality of diet. This would include more data on the micronutrient composition and protein sources of plant versus animal-based foods – not just proportion of fat, carbohydrates, and protein. Such trials should include children, as many adult disorders are seeded as early as infancy or in utero.
Regular physical activity
For decades, surgeon generals’ guidelines have emphasized that daily moderate-to-vigorous aerobic physical activity has both immediate and long-term health benefits. For example, why we age and the rate at which we age – chronological age versus biological age – is determined by multiple molecular processes that are directly influenced by physical activity. And now scientists are gaining a better understanding of the cellular and molecular changes that exercise induces to reduce disease risk.
Research priorities for scientists and physicians include obtaining a deeper understanding of the type, intensity, and frequency of activity, and better insights into the molecular and cellular alterations that occur with exercise.
Restorative sleep
Sleep helps the cells, organs, and entire body to function better. Regular uninterrupted sleep of seven hours per night for adults, eight to 10 hours for teenagers, and 10 or more for children is necessary for good health.
Though understudied, there is evidence that high-quality sleep can reduce inflammation, immune dysfunction, oxidative stress, and epigenetic modification of DNA, all of which are associated with or cause chronic disease.
Therefore, research into the biological mechanisms that underlie the restorative properties of sleep could lead to environmental or population-based and policy approaches to better align our natural sleep patterns with the demands of daily life.
Stress management
Though some stress is beneficial, prolonged or extreme stress can overwhelm the brain and body. Chronic stress increases the risk of cardiovascular disease, irritable bowel disease, obesity, depression, asthma, arthritis, autoimmune diseases, cardiovascular disease, cancer, diabetes, neurological disorders, and obesity.
One of the most powerful mechanisms to reduce stress and enhance resilience is by eliciting a relaxation response using mind-body therapies and cognitive behavioral therapy.
More research is needed to gain a better understanding of how these therapies work.
Addiction reduction and elimination
Many social, economic, and environmental factors have fueled the national rise in substance abuse generally and, most tragically, the opioid epidemic.
Physicians and researchers are beginning to understand the underlying physiology and psychology of addiction.
Yet the continued stigma and disjointed or absent access to services remains a challenge. Clinicians and scientists need to explore how to predict who is more vulnerable to addiction and find ways of preventing it. Treatment that incorporates integrated care focused on all the patient’s needs should be prioritized.
Positive psychology and social connection
Maintaining a positive mindset through the practice of gratitude and forgiveness has a significant impact on psychological and subjective well-being, which are, in turn, associated with physical health benefits.
Social connectivity, namely the quantity and quality of our relationships, has perhaps the most powerful health benefits.
Conversely, social isolation – such as living alone, having a small social network, participating in few social activities, and feeling lonely – is associated with greater mortality, increased morbidity, lower immune system function, depression, and cognitive decline.
Further study is needed to uncover how an individual’s biology and chemistry change for the better through more social interactions.
Inflammation’s role in lifestyle-related diseases
Unhealthy lifestyle behaviors produce a vicious cycle of inflammation. While inflammation is a healthy, natural way the body fights infections, injury, and stress, too much inflammation actually promotes or exacerbates the diseases described above.
The inflammatory response is complex. We have been using machine learning and computer modeling to understand, predict, treat, and reprogram inflammation – to retain the healing elements while minimizing the detrimental more chronic ones. Scientists are unraveling new mechanisms that explain how chronic stress can turn genes on and off.
Overcoming challenges and barriers
We and others who study lifestyle medicine are now discussing how we can leverage all of these approaches to improve clinical studies on the impacts of lifestyle interventions.
At the same time, we and our colleagues realize that there are environmental challenges and barriers that prevent many people from embracing these lifestyle fixes.
There are food deserts where healthier foods are not available or affordable. Unsafe neighborhoods, harmful chemicals and substances create constant stress. Poor education, poverty, cultural beliefs and racial and ethnic disparities and discrimination must be addressed for all people and patients to appreciate and embrace the six “pills.”
The application of lifestyle medicines is particularly important now because unhealthy lifestyles have caused a pandemic of preventable chronic diseases that is now exacerbating the COVID-19 pandemic, which disproportionately afflicts those with these conditions.
Ask your doctor to “prescribe” these six “pills” for a longer and better life. After all, they’re free, work better than or as well as medications, and have no side effects!
Listen to a Podcast with Drs. Vodovotz and Parkinson here.
Illustration: Use the Healthy Eating Plate as an evidence-based guide for creating healthy, balanced meals. ©2011, Harvard University, CC BY-NC.
The Force to Shape an Organ
Carnegie Mellon University’s Biomedical Engineering and Materials Science and Engineering Professor Adam Feinberg, PhD, along with postdoctoral fellow Dan Shiwarski, PhD, and graduate student Joshua Tashman have created a novel biosensor that reveals the mechanobiological forces that shape organ development. Dr. Feinberg is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
These little-understood forces are of increasing interest to medicine and research and the team’s findings offer important insights vital for understanding disease, as well as the future of bioengineered organs.
In this instance, a mechanobiological force refers to the change in shape and size of a tissue resulting from mechanical forces that cells exert on surrounding cells and the extracellular environment. While mechanical forces are an integral component of tissue development, measuring these forces in three dimensions—let alone in living tissue—is extremely complex. The incorporation of physiologic forces, especially those that drive proper development, has been largely overlooked in bioengineered tissue and organ research due to a lack of quantitative information. If researchers could directly measure these forces and the resulting mechanical strains within living tissue during development it could lead to both a critical understanding of fundamental principles, as well as provide strategies for using applied forces to mature engineered tissue.
“With the ability to culture and differentiate large quantities of functional cells from induced pluripotent stem cells, a new era of 3D tissue engineering has evolved to reveal the potential of regenerative medicine,” said lead author Dr. Shiwarski. “However, researchers are now finding out that, in addition to growth factor driven differentiation processes, the inclusion of mechanical forces is essential to stimulate proper cellular organization and tissue function.”
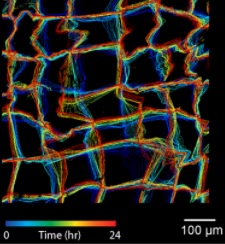
In their recent publication in Nature Communications, Dr. Feinberg’s team worked to develop their novel nanomechanical biosensor (NMBS) as a means for measuring and mapping the effect of mechanobiological forces on cell movement, differentiation, and tissue growth. The NMBS is a fluorescently labeled mesh made from extracellular matrix protein with individual lattice sections measuring from 2-100 micrometers.
By observing the NMBS lattice in 3D over time, the team was able to track microscopic movement between cells and their environment. Using a series of custom image analysis and segmentation techniques they were then able to convert the cell-induced deformations in the NMBS into a map depicting the strain, directionality, and dynamics of the mechanobiological forces at work. Their method was verified through computational simulation, mechanical testing of materials with known properties, and application of the NMBS onto the surface of cells and developing tissue.
Understanding the deformation that mechanobiological forces impart on developing tissue is crucial to recognizing how a collection of cells come together to form an organ. The cells and extracellular matrix are subjected to forces throughout their growth, shaping how they form and assemble into a cohesive system. If researchers can map the mechanobiological forces at play during development, then they can start creating ways to prepare and “train” cells to replicate the natural growth process. As we continue to gain a better understanding of mechanobiological forces and their effect, the NMBS could also potentially prove useful for future research in medicine and cell biology.
“We are really excited that the NMBS technology will provide new insights into the role mechanical forces play in a wide range of biological phenomena, from diseases such as hypertension to the formation of new tissues and organs during development. Ultimately, we hope that this knowledge will help lead to new and improved therapies,” said Dr. Feinberg.
Much like 3D printing techniques also being developed in Feinberg’s lab, the NMBS is a new tool developed to overcome one of the major hurtles standing between modern healthcare and a near endless supply of lab grown replacement organs. Though we may still be decades from realizing that goal, important tools such as this continue to advance tissue engineering for regenerative medicine and change the way we understand the process of organ development.
Illustration: Extracted from the NMBS utilized for 3D strain mapping of drosophila ovariole tissue. Carnegie Mellon University.
Where Applied Biophysics Meets Tissue Engineering and Cellular Therapies
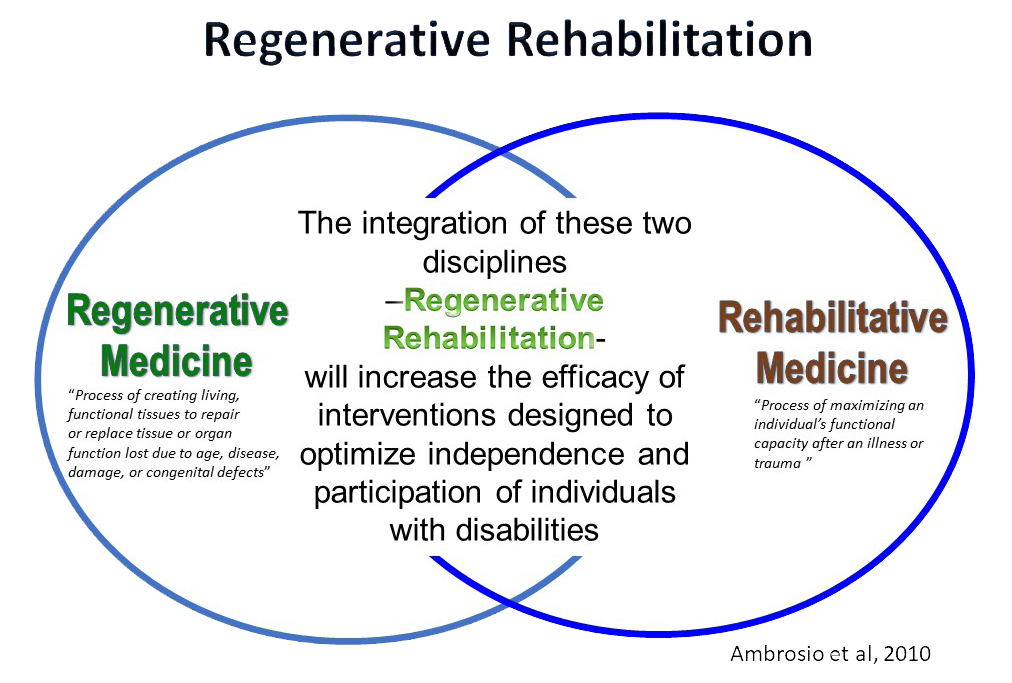
Regenerative medicine focuses on the repair or replacement of tissue lost to injury, disease, or age, primarily via the enhancement of endogenous stem cell function or the transplantation of exogenous stem cells. The focus of rehabilitation science is on the use of mechanical and other stimuli to promote functional recovery. The field of Regenerative Rehabilitation integrates these two approaches, with the ultimate goal of optimizing outcomes. It is where applied biophysics meets tissue engineering and cellular therapies.
McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, MPT, is the Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh. Dr. Ambrosio’s research has the long-term goal of developing Regenerative Rehabilitation approaches to enhance skeletal muscle function with increasing age and in the setting of disease. She is a co-author of the chapter on cell therapies/transplantation in Goodman and Fuller’s Pathology, 5th Edition, by Catherine C. Goodman, MBA, PT, CBP and Kenda S. Fuller, PT, NCS. The authors and associate editor Rolando Lazaro, PT, PhD, DPT, recently shared their insights on the landscape of physical therapy and important topics covered in their new title. Their response to a question re Regenerative Rehabilitation during that conversation follows:
How does regenerative medicine translate to physical rehabilitation management?
Regenerative Rehabilitation has been defined as “the application of rehabilitation protocols and principles together with regenerative medicine therapeutics toward the goal of optimizing functional recovery through tissue regeneration, remodeling, or repair.” The rationale for Regenerative Rehabilitation includes three main areas, including:
For functional tissue regeneration to occur, resident stem cells are dependent upon supporting cues from the microenvironment, or niche. These microenvironmental cues include vascularity, growth factor secretion, and neural signals, for example, all of which serve to direct stem cell fate.
Stem cells “sense” static and dynamic physical aspects of their surroundings. Through a process known as “mechanotransduction,” or the conversion of physical stimuli into chemical responses, extrinsic signals may directly affect stem cell responses, such as gene expression and differentiation into a specific tissue lineage.
Differentiation of stem cells into the tissue target is often a goal of stem cell therapeutics, and physical therapy may serve as an important adjunct therapy to promote functionality of the newly formed tissue. Future studies are needed to investigate the ability of physical therapy protocols to promote hypertrophy of the newly formed myofibers so as to maximize functional outcomes.
Clearly, physical therapists will play a major role in the success of regenerative medicine and tissue engineering approaches in the clinic, much in the same way that successful outcomes following an orthopedic procedure relies on well-defined post-surgical rehabilitation protocols.
Illustration: Ambrosio, et al. 2010.
Regenerative Medicine Patient Runs 5K Race in 2020 After Above-the-Knee Amputation in 2015

Darshit Thakrar, MD, is a California physician who lost his left leg in an accident five years ago but hasn’t let this tragedy be an obstacle when it comes to accomplishing his dreams. The surgical expertise of McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, FACS, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, was instrumental in helping Dr. Thakrar with his plans to run in an upcoming race for which he was training prior to the accident.
After Dr. Thakrar’s above-the-keen amputation, he was experiencing phantom pain in the missing limb. Phantom pain involves the sensation of pain in a part of the body that has been removed. Symptoms can include: Onset within the first few days of amputation. Comes and goes or is continuous. Often affects the part of the limb farthest from the body, such as the foot of an amputated leg. May be described as shooting, stabbing, boring, squeezing, throbbing, or burning. Sometimes feels as if the phantom part is forced into an uncomfortable position. May be triggered by pressure on the remaining part of the limb or emotional stress.
To alleviate the phantom pain Dr. Thakrar was experiencing, Dr. Rubin first removed the scar tissue from the original amputation site and then placed fat, excised from another part of Dr. Thakrar’s body, to this same site. The fat provided additional soft tissue padding around the injured nerve endings of Dr. Thakrar’s leg. Most importantly, the fat tissue includes a population of naturally occurring stem cells which help with healing. Dr. Rubin initially developed and conducted this tissue reconstruction process for injured military personnel.
“I was so delighted,” Dr. Rubin told PEOPLE (The TV Show!). “I mean, what a wonderful, wonderful outcome, to be able to go from essentially barely getting through your day and barely getting around to running a 5K… That was just wonderful. How amazing to see him reach that goal of actually completing a 5K. It’s just terrific.”
Watch Drs. Thakrar and Rubin’s story here.
Dr. Mario Solari Receives MTF Biologics Allograft Tissue Research Grant

MTF Biologics, a global nonprofit organization that saves and heals lives by advancing tissue and organ donation, transplantation, and research, has awarded over $1 million in funding to 11 researchers across the nation through its 2020 Extramural Research Grants Program. One of the 11 funding recipients is McGowan Institute for Regenerative Medicine affiliated faculty member Mario Solari, MD, Assistant Professor in the Departments of Plastic Surgery and Otolaryngology within University of Pittsburgh’s School of Medicine, and Director of the Vascularized Composite Allotransplantation and Microsurgery (VCAM) Laboratory, for his project entitled “Engineering Vascularized Soft Tissues for Definitive Complex Wound Reconstruction.” Funding for this project is the MTF Biologics Allograft Tissue Research Grant, administered by the Plastic Surgery Foundation.
MTF Biologics is a global nonprofit organization that saves and heals lives by advancing tissue and organ donation, transplantation, and research. They provide service, resources, and expertise to donors and their loved ones who give the gift of life, people who depend on tissue and organ transplants, healthcare providers, and clinicians and scientists.
Congratulations, Dr. Solari!
Dr. Jason Shoemaker Awarded Two NIAID Grants

McGowan Institute for Regenerative Medicine affiliated faculty member Jason Shoemaker, PhD, Assistant Professor in the Departments of Chemical and Petroleum Engineering and Computational and Systems Biology, recently received National Institutes of Health funding for two R21s. Both of these R21s will be awarded from the National Institute of Allergy and Infectious Diseases.
The first is entitled “Sex-Specific Immune Responses to Severe Influenza Virus Infection” and is for $450,000 where Dr. Shoemaker is the PI.
The abstract follows:
Men and women experience influenza virus infection differently, with women often experiencing a more severe infection. Lung immunopathology is a major contributing factor to influenza virus disease severity and has been linked to differential disease outcomes in men and women. The differences between male and female immune responses during infection are the immune response dynamics, i.e., the speed and magnitude of the reaction of key immune molecules and cells to the virus. These dynamics are regulated by the molecular and cellular interactions that comprise the lung immune system, and it has been shown that lung immune dynamics can be altered in women by altering levels of circulating sex steroids (estradiol) to affect lung inflammation and overall infection severity. Here, we propose an experimental and computational modeling study to quantify the differential immune kinetics that drive the distinct lung immunopathological outcomes observed between men and women. Human male and female infection outcomes have been recapitulated in mouse models. We will infect male and female mice with a moderate pandemic H1N1 virus and a deadly avian influenza virus, collect dynamic immunologic and hormone data, and train mathematical models to the data to quantify the immune kinetics regulating the differential dynamic lung immune responses observed between the sexes. The immunologic data will include major cytokines and immune cells with established significance to virus clearance and respiratory tissue inflammation. Successful completion of the research program will provide the first sex-specific mathematical models of influenza-induced immune responses, the first mathematical models of the avian influenza-induced immune response, and the first mathematical models quantifying the impact of sex hormones on lung immune regulation in females. By quantifying which immune kinetics are different between males and females during moderately and severely pathogenic infections, we will generate novel hypotheses on the molecular/cellular origins of lung immunopathology during influenza infection and provide quantitative evidence on whether mechanisms promoting severe lung pathology are dependent on sex, virulence of the virus, or both factors. And, quantifying the relationship between sex hormones and lung immune activity in females may provide insight into the mechanisms associated with the increased susceptibility experienced by pregnant women during severe influenza virus infection.
The second is entitled “Mathematical Modeling of Influenza Severity in Outbred Mice” and is also for $450,000 where Dr. Shoemaker is a Co-PI. The abstract follows:
In the United States, pulmonary influenza infection occurs annually in 5-20% of the population with mortality in the range of 30,000 deaths. The recent 2009 influenza H1N1 pandemic illustrated the potential for higher infection rates, which were reported to be as high as 45% in certain age groups. The 2017-18 influenza season had the highest pediatric mortalities since the 2009 pandemic. Influenza infection is known to result in a broad spectrum of disease phenotypes in humans, although severe pneumonia is relatively rare. Despite this, severe disease often requires advanced supportive care in the young, including previously healthy children. Host factors involved in determining the outcome of influenza infection are unclear and children are known to be at higher risk of severe disease. First life exposure to influenza is also thought to dictate life-long immunity. Little is known about the effects of young age and gender on influenza responses and severity. This underscores the importance of understanding influenza pathogenesis in a pediatric population. Influenza pathogenesis is likely mediated in large part by exuberant inflammatory host responses in the lung. It is likely that predictive soluble inflammatory mediators are present in severe infection. Further, predictive biomarkers or mathematical models of influenza pneumonia severity would enhance clinical decision making and patient care. We propose that machine learning and mathematical modeling of host immune endpoints will define a molecular fingerprint of severe influenza pneumonia in juveniles. This hypothesis will be tested in two Aims. Aim 1 will focus on characteristic molecular pathways related to influenza severity in juvenile animals, using outbred mice. We will utilize machine learning and new mathematical approaches for pathway and biomarker selection. Aim 2 will test mathematical models of influenza pathogenesis to elucidate new mechanisms that drive lung injury. The overall goal of the proposed study is to identify novel biomarkers and mechanistic models of influenza pneumonia severity that can be applied to children. To accomplish this, we will use a broad, exploratory, and unbiased approach. Candidate biomarkers and pathways would then be evaluated in future mechanistic and translational studies in mice and humans.
Congratulations, Dr. Shoemaker!
Cystic Fibrosis Foundation Announces Research Agreements to Advance Its Path to a Cure

The Cystic Fibrosis Foundation announced $1.7 million in new research funding to drive progress on its Path to a Cure. The awards, to seven academic institutions and two companies, will support focused research into key scientific challenges associated with developing therapies to address the underlying cause of disease for all people with CF, regardless of their underlying mutation.
McGowan Institute for Regenerative Medicine affiliated faculty member Adam Feinberg, PhD, Professor in the Departments of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University, was one of the nine awardees. Dr. Feinberg will further develop and test a platform capable of overcoming the innate immune defenses in the lungs to successfully deliver a potential new genetic-based therapy to the airway epithelium, the tissue that lines the airways.
“There has been an explosion of scientific progress in novel technologies with the potential to benefit all people with CF, yet significant additional research will be required to move these advances out of the lab and safely to patients,” said William Skach, MD, executive vice president and chief scientific officer of the Cystic Fibrosis Foundation. “These awards reflect important investments into the foundational research that is needed to advance curative therapies for all people with cystic fibrosis.”
The Foundation is now accepting proposals for research funding in the 2021 calendar year. More information about funding opportunities is available on cff.org.
Congratulations, Dr. Feinberg!
Phase III Clinical Trial Data of Gene Therapy to Treat Leber Hereditary Optic Neuropathy Published

Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 800-1,200 new patients who lose their sight every year in the United States and the European Union.
Co-founded by José-Alain Sahel, MD, a McGowan Institute for Regenerative Medicine affiliated faculty member, GenSight Biologics, a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, announced recently that the journal Science Translational Medicine has published results from the REVERSE pivotal Phase III clinical trial of LUMEVOQ® gene therapy in ND4 LHON subjects along with key results from a non-human primate study investigating the contralateral effect of the gene therapy. The paper published under the title “Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy” is the first peer-reviewed article based on Phase III clinical trial data to document sustained and clinically meaningful bilateral improvement in visual outcomes from a unilateral injection of a gene therapy.
“Our study provides great hope for treating this blinding disease in young adults,” said Dr. Sahel, co-corresponding author and Director of the Institut de la Vision (Sorbonne-Université/Inserm/CNRS), Paris, France, where LUMEVOQ®’s underlying mitochondrial targeting technology was developed. “Our approach isn’t just limited to vision restoration: other mitochondrial diseases could be treated using the same technology,” added Dr. Sahel, who is also Chairman of the Department of Ophthalmology at Centre Hospitalier National d’Ophtalmologie des XV-XX, Paris, France, and Professor and Chairman of the Department of Ophthalmology at the University of Pittsburgh School of Medicine and UPMC (University of Pittsburgh Medical Center).
Treated eyes showed a mean improvement in best-corrected visual acuity (BCVA) of 15 letters on an ETDRS chart, representing three lines of vision, while a mean improvement of 13 letters was observed in the sham treated eyes. As some patients were still in the dynamic phase of the disease process upon enrolment, the visual gain from the nadir (worst BCVA for each eye) was even larger, reaching 28.5 letters for the treated eyes and 24.5 letters for sham-treated eyes.
The findings from the REVERSE trial and the non-human primate study were key components of the data package submitted by GenSight Biologics in September 2020 to the European Medicines Agency when it applied for marketing authorization for LUMEVOQ® as treatment for patients with visual loss due to LHON caused by a confirmed mutation in the ND4 mitochondrial gene. The agency’s decision is expected in Q4 2021.
“As someone who treats these young patients, I get very frustrated about the lack of effective therapies,” said Dr Sahel. “These patients rapidly lose vision in the course of a few weeks to a couple of months. Our study provides a big hope for treating this blinding disease in young adults.”
Stress May Awaken Dormant Cancer Cells
Sometimes cancer comes back long after the original tumor has been treated and removed. This is called recurrent cancer. Cancer can recur in the same place as the original tumor or in other places in the body if the tumor cells spread. Cancerous cells can lie dormant for years. But what triggers these cells to reawaken hasn’t been well understood.
Past studies have linked chronic stress with cancer progression. To investigate whether stress can awaken dormant tumor cells, a research team led by Dmitry Gabrilovich, MD, PhD, of AstraZeneca and Valerian Kagan, PhD, DSc, of the University of Pittsburgh and Sechenov University developed mouse models with dormant tumors. Their study was funded in part by NIH’s National Cancer Institute (NCI). Results were published in Science Translational Medicine.
The team tested the effects of several stress hormones—including cortisol, epinephrine, norepinephrine, and serotonin—on dormant tumor cells taken from the mice. They found that neutrophils, a type of disease-fighting immune cell, were activated by the stress hormones. The neutrophils then produced inflammation-inducing proteins called S100A8 and S100A9.
Further experiments showed that these proteins were needed to reactivate the dormant tumor cells. However, they didn’t directly affect dormant tumor cells. The presence of S100A8/A9 altered certain lipids (fats) and led to their accumulation in the neutrophils. These lipids interacted with the dormant tumor cells, leading to tumor cell reactivation and new tumors.
Periodically stressing mice with dormant tumors led to tumor growth. However, giving the mice a beta blocker, which blocks stress hormone, prevented tumor cell reactivation.
To determine whether humans have a similar stress-induced response, the researchers tested blood samples taken from 80 people with non-small cell lung cancer. They compared levels of norepinephrine and S100A8/A9 in those who never had a cancer recurrence or had a late recurrence with those who had an early recurrence (within 33 months). Patients who had an early recurrence showed higher levels of the molecules.
“Our data suggest that stress hormone levels should be monitored in patients recovering from cancer and that managing stress to keep those hormones at bay would be beneficial to prolong remission,” says Michela Perego, PhD, of The Wistar Institute, who is first author of the paper.
There are multiple factors that come together to cause a recurrence of cancer. These results show that stress hormone levels are one that might be monitored and reduced in standard cancer treatment. The findings also suggest new therapeutic approaches to investigate for preventing cancer relapse.
At the University of Pittsburgh, Dr. Kagan is a Professor and Vice-Chairman in the Department of Environmental and Occupational Health as well as a Professor in the Department of Pharmacology and Chemical Biology, the Department of Radiation Oncology, and the Department of Chemistry. He is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Illustration: Activation of neutrophils, a type of disease-fighting immune cell shown above, by stress hormones may lead dormant cancer cells to awaken. somersault18:24 /iStock/Getty Images Plus.
Dr. Youngjae Chun Receives Second-Year Research Funding from The Children’s Heart Foundation

McGowan Institute for Regenerative Medicine affiliated faculty member Youngjae Chun, PhD, associate professor of industrial engineering and bioengineering at the University of Pittsburgh Swanson School of Engineering, will receive second-year research funding as part of more than $735,000 from The Children’s Heart Foundation, the nation’s leading organization dedicated to funding congenital heart defect (CHD) research.
Dr. Chun is one of three researchers receiving second-year funding for research that, according to the Foundation, has made significant progress this year. His research is on “A Self-Growing Percutaneous Heart Valve Frame to Treat Congenital Heart Disease.” His research efforts will help experts learn more about the life-long care needs of individuals living with CHDs and how to continue to improve their overall quality of life.
Announced in March 2020, Dr. Chun’s research focuses on developing a new type of metallic frame for pediatric heart valves that could not only be placed by a minimally invasive catheter-based procedure but would also grow with the child, eliminating the need for follow-up surgeries.
The Foundation will fund CHD research and scientific collaborations this year across four key initiatives:
- independent research funded by the Foundation,
- collaborative research with the American Heart Association through joint Congenital Heart Defect Research Awards,
- funding the American Academy of Pediatrics’ Pediatric Cardiology Research Fellowship Award, and
- funding Cardiac Networks United (CNU), a national pediatric and congenital cardiovascular research network.
Every 15 minutes, a baby is born with a congenital heart defect, making CHDs America’s most common birth defect. The Children’s Heart Foundation’s mission is to advance the diagnosis, treatment, and prevention of CHDs by funding the most promising research. Since 1996, the Foundation has been a proven leader, funding nearly $14 million of CHD research and scientific collaborations.
METAvivor Translational Research Award Received by Dr. Steffi Oesterreich

McGowan Institute for Regenerative Medicine affiliated faculty member Steffi Oesterreich, PhD, Professor of Pharmacology and Chemical Biology at the University of Pittsburgh Cancer Institute, is a recipient of a METAvivor Translational Research Award. Dr. Oesterreich’s project is entitled “Targeting mesothelial – tumor cell interaction to treat invasive lobular cancer metastases.” This project was one of the 23 new grant awards for metastatic breast cancer research totaling $3,650,000.
Metastatic breast cancer (also known as stage IV or advanced stage cancer) is the spread of breast cancer to other parts of the body — most commonly to the bones, liver, lungs, and/or brain. Approximately 30% of breast cancer patients metastasize, with the mean survival after diagnosis being 33 months. In the U.S., only 2-5% of all cancer research funds are dedicated to stage IV cancer research – yet 98% of all breast cancer deaths are caused by a metastasis.
METAvivor Research and Support Inc. is an Annapolis-based, volunteer-led, non-profit organization founded by metastatic breast cancer (MBC) patients in 2009. The organization’s main focus is to fund critical research that will lead to advances in treatment options, quality of life and survival for patients diagnosed with MBC. Since 2009, METAvivor has awarded 129 research grants totaling $17,250,000. METAvivor is the only national organization with a peer-reviewed grant program aimed at exclusively funding MBC research, and 100% of all donations go to fund research.
Congratulations, Dr. Oesterreich!
AWARDS AND RECOGNITION
Dr. Stephen Badylak Named Fellow, Biomaterials Science and Engineering

McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, was recently inducted as a Fellow, Biomaterials Science and Engineering (FBSE), by the International Union of Societies for Biomaterials Science and Engineering (IUSBSE) and was recognized during a virtual ceremony at the World Biomaterials Congress on December 11, 2020. The honorary status of FBSE was established in April 1992 after the constituent biomaterials societies of the World Biomaterials Congress, now the IUSBSE, recognized the need for the public recognition of those of their members who have gained a status of excellent professional standing and high achievements in the field of biomaterials science and engineering.
Dr. Badylak is a Professor in the Department of Surgery, University of Pittsburgh, and Director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute. His major research interests include:
- tissue engineering and regenerative medicine
- biomaterials and biomaterial/tissue interactions
- developmental biology and its relationship to regenerative medicine
- relationship of the innate immune response to tissue regeneration
- biomedical engineering as it relates to device development and biomaterials
- clinical translation of regenerative medicine
The IUSBSE is a body that brings together national and multi-national groups dedicated to the advancement of biomaterials, surgical implants, prosthetics, artificial organs, tissue engineering, and regenerative medicine. It currently includes members from Australia, Canada, China, Chinese Taipei, Europe, India, Japan, Korea, South America, and the United States of America.
Congratulations, Dr. Badylak!
Illustration: The Badylak Laboratory.
Dr. William Wagner Named Society For Biomaterials 2021 Founders Award Recipient

McGowan Institute for Regenerative Medicine director William Wagner, PhD, named the 2021 Founders Award recipient from the Society For Biomaterials (SFB). The Founders Award is based on long-term, landmark contributions to the discipline of biomaterials. Dr. Wagner will be presented with the award at the virtual SFB 2021 Annual Meeting & Exposition being held April 20-23, 2021, during which he will make a plenary address. As the recipient of this prestigious recognition, he will receive a cash award and free registration to the virtual 2021 SFB Annual Meeting & Exposition.
Dr. Wagner is a Distinguished Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. He also currently serves as Chairman of the Tissue Engineering and Regenerative Medicine International Society (TERMIS) – Americas, the Deputy Director of the NSF Engineering Research Center on “Revolutionizing Metallic Biomaterials,” and Chief Scientific Officer of the Armed Forces Institute of Regenerative Medicine. He holds a BS (Johns Hopkins University) and PhD (University of Texas) in Chemical Engineering.
Dr. Wagner is the Founding Editor and Editor-in-Chief of one of the leading biomaterials and biomedical engineering journals, Acta Biomaterialia, and currently serves on the editorial boards of the Journal of Biomedical Materials Research Part A, Biotechnology and Bioengineering, Organogenesis, Experimental Biology & Medicine, and the Journal of Tissue Engineering and Regenerative Medicine. Dr. Wagner is a past president of the American Society for Artificial Internal Organs (ASAIO; 2010-2011) and has served on the Executive Board of the International Federation of Artificial Organs (IFAO). He is a fellow and former vice president of the American Institute for Medical and Biological Engineering (AIMBE; 2000) and has been elected a fellow of the Biomedical Engineering Society (2007), the International Union of Societies for Biomaterials Science and Engineering (2008), the American Heart Association (2001), and TERMIS (2015). He has served as Chairman for the Gordon Research Conference on Biomaterials: Biocompatibility & Tissue Engineering as well as for the Biomedical Engineering Society Annual Meeting, ASAIO, and the First World Congress of TERMIS. He was previously recognized by selection to the “Scientific American 50”, the magazine’s annual list recognizing leaders in science and technology from the research, business, and policy fields. In 2011 he was awarded the Society for Biomaterials Clemson Award for Applied Research, in 2012 he received the Chancellor’s Distinguished Research Award from the University of Pittsburgh, and in 2013 he received the TERMIS Senior Scientist Award. He has served on numerous NIH and NSF study sections, is a member of the NIH College of Reviewers, and has been a member of external review committees for national and international organizations focused on bioengineering and regenerative medicine. His research has generated numerous patents and patent filings that have resulted in licensing activity, the formation of a company that has reached clinical trials, and University of Pittsburgh Innovator Awards in 2007, 2008, 2009, 2010, 2014, and 2018. In 2017, he was inducted as a Fellow in the National Academy of Inventors (NAI), and in 2018, he was named Inventor of the Year, by the Pittsburgh Intellectual Property Law Association.
Dr. Wagner’s research interests are generally in the area of cardiovascular engineering with projects that address medical device biocompatibility and design, hypothesis-driven biomaterials development, tissue engineering, and targeted imaging. His research group has been comprised of graduate students in Bioengineering and Chemical Engineering as well as post-doctoral fellows with backgrounds in surgery, polymer chemistry, or engineering. Dr. Wagner and his group enjoy working across the spectrum from in vitro to clinical studies. The McGowan Institute and the University of Pittsburgh Medical Center are uniquely positioned to allow such broad-based projects to flourish and complement one another. Researchers within Dr. Wagner’s group are afforded the opportunity to observe first-hand the clinical successes and failures of currently employed cardiovascular devices while concurrently working on projects that attempt to describe the current modes of failure, test solutions for the current device shortcomings, or develop technologies that may find application as future cardiovascular therapies. The front-line experience afforded by the clinical environment has proven invaluable in the learning experience of group members, not to mention the input such experience has on the creative environment.
The recipient of the Founders Award is selected from formal nominations submitted accompanied by the curriculum vitae of the candidate, two supporting letters of recommendation, and a brief bio-sketch. The awardee will be requested to submit a research or review manuscript for publication consideration in the Journal of Biomedical Materials Research. The Society reserves the right to publish the manuscript in the Journal of Biomedical Materials Research or Applied Biomaterials.
Congratulations, Dr. Wagner!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#217 –– Drs. Yoram Vodovotz and Michael Parkinson discuss their work in Lifestyle Medicine and its impact on the immune system.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Jingyao Wu, Leila J. Mady, Abhijit Roy, Ali Mübin Aral, Boeun Lee, Feng Zheng, Toma Catalin, Youngjae Chun, William R. Wagner, Ke Yang, Humberto E. Trejo Bittar, David Chi & Prashant N. Kumta
Title: In-vivo efficacy of biodegradable ultrahigh ductility Mg-Li-Zn alloy tracheal stents for pediatric airway obstruction
Summary: Pediatric laryngotracheal stenosis is a complex congenital or acquired airway injury that may manifest into a potentially life-threatening airway emergency condition. Depending on the severity of obstruction, treatment often requires a combination of endoscopic techniques, open surgical repair, intraluminal stenting, or tracheostomy. A balloon expandable biodegradable airway stent maintaining patency while safely degrading over time may address the complications and morbidity issues of existing treatments providing a less invasive and more effective management technique. Previous studies have focused on implementation of degradable polymeric scaffolds associated with potentially life-threatening pitfalls. The feasibility of an ultra-high ductility magnesium-alloy based biodegradable airway stents was demonstrated for the first time. The stents were highly corrosion resistant under in vitro flow environments, while safely degrading in vivo without affecting growth of the rabbit airway. The metallic matrix and degradation products were well tolerated by the airway tissue without exhibiting any noticeable local or systemic toxicity.
Source: Communications Biology 3, 787 (2020).
GRANT OF THE MONTH
PI: William R. Wagner
Title: Support for Proof-of-Concept Studies in Regenerative Medicine
Description: This proposal provides support for proof-of concept studies for advancing the state-of-the-art in regenerative medicine. Successful studies will be positioned to compete for advanced studies with funding from Federal agencies. The proposal includes projects involving both clinical and academic researchers all of whom are active in the growing field of Regenerative Medicine.
Source: Commonwealth of Pennsylvania, Department of Health
Term: 7/1/20 – 12/31/21 (Estimated)
Amount: $1,300,000
