January 2018 | VOL. 17, NO. 1| www.McGowan.pitt.edu
Gene Therapy Reverses Autoimmune Diabetes in Mice

Reversing autoimmune type 1 diabetes without immunosuppression has proven to be extremely difficult, but in a study published in Cell Stem Cell, researchers at Children’s Hospital of Pittsburgh of UPMC and the University of Pittsburgh School of Medicine report achieving that outcome in mice using gene therapy.
In type 1 diabetes, the body mistakenly recognizes the insulin-producing ‘beta’ cells in the body as foreign and kills them, resulting in high blood sugar levels. Patients require lifelong insulin therapy either through injections or an insulin pump. The current study however, represents a major advance in efforts to develop a long-term therapeutic approach by stimulating the body’s own pancreatic cells to produce insulin.
“We have shown for the first time that gene therapy can be specifically and effectively targeted to reverse autoimmune diabetes in mice without the use of any immunosuppressant drugs,” said George Gittes, MD, chief of pediatric surgery at Pitt’s School of Medicine, the Benjamin R. Fisher Chair in Pediatric Surgery at Children’s Hospital, and an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Dr. Gittes and his team reprogrammed non-insulin producing pancreatic ‘alpha’ cells into insulin producing beta cells by introducing two genes with the help of a virus commonly used in gene therapy. By using a recently developed method called ‘pancreatic intraductal viral infusion,’ the researchers directly delivered the therapeutic virus to the pancreas, thus using a much smaller amount of virus when compared to traditional gene therapy injections and reducing the chances for side effects.
When tested in a mouse model in which diabetes had been induced by killing off beta cells with a toxin, Dr. Gittes and his team found that the gene therapy reversed the diabetes by reprogramming alpha cells into beta cells, which produced insulin and returned blood sugar levels to normal.
The team then evaluated the approach in a mouse model of autoimmune type 1 diabetes. While these mice normally die within approximately five weeks of the onset of high blood sugar due to the persistence of excessive blood sugar, the gene therapy resulted in conversion of alpha cells into beta cells and prolonged blood sugar control for about 16 weeks in the mice tested.
Importantly, the newly reprogrammed cells were not destroyed due to an autoimmune reaction for approximately four months, a significantly longer time when compared to other methods such as beta cell transplantation in which the cells are killed immediately. The reason behind the protection from autoimmune attack is still unclear, but is likely linked to the location of the newly reprogrammed beta cells in the pancreas, according to the authors.
“This method to deliver a gene therapy directly to the pancreas could easily be applied to humans, as similar pancreatic injections are routinely performed as part of a non-surgical endoscopic procedure,” said Dr. Gittes.
The researchers currently are testing the therapy in a non-human primate model, and if proven effective, could soon initiate clinical trials.
RESOURCES AT THE MCGOWAN INSTITUTE
February Histology Special
The McGowan Institute Histology Core wants to be your Valentine this month with big savings that will warm your heart. Bring your cardiac tissue to the McGowan Histology Core and receive 25% off your entire order in the month of February, when you mention this ad.
From our hearts to yours, you won’t beat these prices, or our rapid turnaround times. Race in today for top quality results!!
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
EVENTS
REGISTRATION OPEN-2018 McGowan Institute Retreat

March 5-6, 2018
Program Highlights:
- State of the Institute Address- William R. Wagner, PhD-Director
- McGowan Institute for Regenerative Medicine Distinguished Lecture, Dr. Molly Shoichet, University of Toronto: “Regenerative Strategies in the Central Nervous System”
New this Year!
NEW FORMAT for breakout sessions: Combination of lectures and audience engagement
- Topics Areas include: Cancer Immunotherapy, Reproductive Tissue Engineering, Additive Manufacturing, Drug Delivery, Craniomaxillofacial Tissue Engineering, Pediatric research-focused topics, and more
Pop-Up Workshops!
- Regenerative Cell and Tissue Therapies at UPMC- Organized by Dr. Kokai and Dr. Rubin
- Early Stage Commercial Translations- Organized by Don Taylor, PhD and the SciVelo Team
- Device Prototyping- Organized by SmithWise
Highlights Include:
- Pittsburgh Biomedical Breakfast
- Roundtable Lunch-Special Interest Topics *trainee focused
- Poster Competition and People’s Choice Award *trainee focused
- Rapid Fire Presentations *trainee focused
- Closing Banquet
Registration and abstract submission site will open January 15th, 2018; Poster session abstracts must be submitted by Febrary 1st to be eligible for a poster session award
Join us on March 5-6, 2018 at the University Club for the 17th Annual McGowan Institute for Regenerative Medicine Scientific Retreat. The program committee under the leadership of Julie Phillippi, PhD, Patrick Cantini, and Christopher Bettinger, PhD has designed an exciting program.
 Regenerative Medicine Summer School 2018
Regenerative Medicine Summer School 2018
Bryan Brown, PhD has announced the 5th Annual Regenerative Medicine Summer School- June 3-9, 2018
Objective: To provide national and regional students with a week-long didactic and experiential learning experience addressing the science and engineering related to the multidisciplinary field of regenerative medicine.
Target Audience: Undergraduates, enrolled in a science or engineering program that will have completed their 3rd year of study; exceptional candidates who will have completed their 2nd year of undergraduate study will be considered.
Venue: McGowan Institute-Pittsburgh, PA: June 3-9, 2018
Students who are interested in summer internships in addition to the summer school should apply by January 31, 2018.
SCIENTIFIC ADVANCES
One Week. Two Authors. Two Journal Covers.
In one week in December 2017, Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and the William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology, and Riccardo Gottardi, PhD, Research Assistant Professor, Center for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, were co-authors on two publications that were featured on the respective journal covers. The McGowan Institute for Regenerative Medicine congratulates these affiliated faculty members on this significant accomplishment. Details follow.
 Modern Therapeutic Approaches for Noninfectious Ocular Diseases Involving Inflammation
Modern Therapeutic Approaches for Noninfectious Ocular Diseases Involving Inflammation
Michelle L. Ratay, Elena Bellotti, Riccardo Gottardi, and Steven R. Little
Advanced Healthcare Materials; 2017 Dec;6(23).
Dry eye disease, age-related macular degeneration, and uveitis are ocular diseases that significantly affect the quality of life of millions of people each year. In these diseases, the action of chemokines, proinflammatory cytokines, and immune cells drives a local inflammatory response that results in ocular tissue damage. Multiple therapeutic strategies are developed to either address the symptoms or abate the underlying cause of these diseases. Herein, the challenges to deliver drugs to the relevant location in the eye for each of these diseases are reviewed along with current and innovative therapeutic approaches that attempt to restore homeostasis within the ocular microenvironment.
 Programmed Platelet Derived Growth Factor‐BB and Bone Morphogenetic Protein‐2 Delivery from a Hybrid Calcium Phosphate/Alginate Scaffold
Programmed Platelet Derived Growth Factor‐BB and Bone Morphogenetic Protein‐2 Delivery from a Hybrid Calcium Phosphate/Alginate Scaffold
Bayer EA, Jordan J, Roy A, Gottardi R, Fedorchak MV, Kumta PN, Little SR.
Tissue Engineering Part A; 2017 Dec;23(23-24):1382-1393.
Bone tissue engineering requires the upregulation of several regenerative stages, including a critical early phase of angiogenesis. Previous studies have suggested that a sequential delivery of platelet-derived growth factor (PDGF) to bone morphogenetic protein-2 (BMP-2) could promote angiogenic tubule formation when delivered to in vitro cocultures of human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs). However, it was previously unclear that this PDGF to BMP-2 delivery schedule will result in cell migration into the scaffolding system and affect the later expression of bone markers. Additionally, a controlled delivery system had not yet been engineered for programmed sequential presentation of this particular growth factor. By combining alginate matrices with calcium phosphate scaffolding, a programmed growth factor delivery schedule was achieved. Specifically, a combination of alginate microspheres, alginate hydrogels, and a novel blend of resorbable calcium phosphate-based cement (ReCaPP) was used. PDGF and BMP-2 were sequentially released from this hybrid calcium phosphate/alginate scaffold with the desired 3-day overlap in PDGF to BMP-2 delivery. Using a three-dimensional coculture model, we observed that this sequence of PDGF to BMP-2 delivery influenced both cellular infiltration and alkaline phosphatase (ALP) expression. It was found that the presence of early PDGF delivery increased the distance of cell infiltration into the calcium phosphate/alginate scaffolding in comparison to early BMP-2 delivery and simultaneous PDGF+BMP-2 delivery. It was also observed that hMSCs expressed a greater amount of ALP+ staining in response to scaffolds delivering the sequential PDGF to BMP-2 schedule, when compared with scaffolds delivering no growth factor, or PDGF alone. Importantly, hMSCs cultured with scaffolds releasing the PDGF to BMP-2 schedule showed similar amounts of ALP staining to hMSCs cultured with BMP-2 alone, suggesting that the sequential schedule of PDGF to BMP-2 presentation promotes differentiation of hMSCs toward an osteoblast phenotype while also increasing cellular infiltration of the scaffold.
Congratulations, all!
Uncovering the Power of Glial Cells
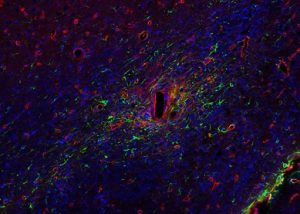
Implanted devices send targeted electrical stimulation to the nervous system to interfere with abnormal brain activity, and it is commonly assumed that neurons are the only important brain cells that need to be stimulated by these devices. However, research published in Nature Biomedical Engineering reveals that it may also be important to target the supportive glial cells surrounding the neurons.
The collaboration was led by Erin Purcell, PhD, assistant professor of biomedical engineering at Michigan State University; Joseph Salatino, Dr. Purcell’s graduate student researcher; Kip A. Ludwig, PhD, associate director of technology at Mayo Clinic; and McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, assistant professor of bioengineering at the University of Pittsburgh’s Swanson School of Engineering.
“Glial cells are the most abundant in the central nervous system and critical to the function of the neuronal network,” Dr. Kozai says. “The most obvious function of glial cells has been related to their role in forming scar tissue to prevent the spread of injury and neuronal degeneration, but so much about their role in the brain is unknown.”
The study, “Glial responses to implanted electrodes in the brain” (doi:10.1038/s41551-017-0154-1) suggests that these glial cells are more functional than previously thought. “From providing growth factor support and ensuring proper oxygen and nutrient delivery to the brain to trimming of obsolete synapses and recycling waste products, recent findings show that glial cells do much more to ensure brain activity is optimized,” Dr. Kozai says.
The slow, dim signals of glial cells are much more difficult to detect than the vibrant electrical activity of neurons. New advancements in technology allows researchers like Dr. Kozai to detect the subtleties of glial cell activity, and these observations are shedding new light on current issues plaguing implant devices and the treatment of neurological disease.
Dr. Kozai explains, “Dysfunction in glial cells has been implicated as a cause and/or major contributor to an increasing number of neurological and developmental diseases. Therefore, it stands to reason that targeting these glial cells (in lieu of or in combination with neurons) may dramatically improve current treatments.”
Dr. Kozai’s lab is currently working with Franca Cambi, MD, PhD, professor of neurology at Pitt, on a project to understand the role of another type of glial cell on brain injury and neuronal activity. “Oligodendrocyte Progenitor Cells,” or OPCs, are progenitor cells—similar to stem cells—that have the capacity to differentiate during tissue repair.
Dr. Kozai believes that it is a pivotal time to investigate these cells and recognizes Dr. Ben Barres, an acclaimed neuroscientist at Stanford University, who made crucial discoveries in glial cell research. Dr. Kozai said, “We lost a great scientist and pioneer in this field of neuroscience. Professor Ben Barres really uncovered the importance of these glial cells on brain injuries and diseases. We have to keep pushing to see how we can improve current treatment by fixing these under-appreciated brain cells.”
Illustration: A depiction of the brain glial cell response towards site injury upon insertion of neural interface probe track (rectangular hole), which disrupts the maintenance of their important regulatory roles. (Image by TDY Kozai/BionicLab.org)
Dr. John Kellum Hails FDA Approval of Drug as a Major Advance in Treatment for Septic or Distributive Shock

La Jolla Pharmaceutical Company announced recently that the U.S. Food and Drug Administration (FDA) has approved GiaprezaTM (angiotensin II) to increase blood pressure in adults with septic or other distributive shock.
“Vasopressors are critical to treat patients with shock. The critical care community now has another tool to use,” said McGowan Institute for Regenerative Medicine affiliated faculty member John A. Kellum, MD, Director of Center for Critical Care Nephrology, Vice Chair for Research, and Professor of Critical Care Medicine, University of Pittsburgh. “The approval of angiotensin II represents a major advance in the treatment of patients with septic or distributive shock.”
Distributive shock is the most common type of shock in the inpatient setting, affecting approximately one-third of intensive care unit patients. There are approximately 800,000 distributive shock cases in the United States per year. Of these cases, an estimated 90% are septic shock patients. Approximately 300,000 do not achieve adequate blood pressure response with current standard therapy. The inability to achieve or maintain adequate blood pressure results in inadequate blood flow to the body’s organs and tissue and is associated with a mortality rate exceeding most acute conditions requiring hospitalization.
La Jolla plans to make GiaprezaTM available for patients in the U.S. in March 2018.
Review Lays out Recommendations, Calls for Research to Improve Post-Hospitalization Sepsis Outcomes

Despite improvement in the rates of people dying of sepsis in the hospital, the condition is still a leading cause of hospital readmissions and costs, as well as long-term disabilities and impairments, prompting University of Pittsburgh and University of Michigan (U-M) medical scientists to develop thorough recommendations for post-hospital recovery care and future clinical trials.
The review, published in the Journal of the American Medical Association, calls for a significant focus on developing an evidence-based approach to optimal care for the 14 million patients worldwide who survive hospitalization for sepsis each year.
“Current treatment guidelines emphasize interventions that reduce short-term mortality, but with little information on strategies to minimize physical disability, cognitive impairment, or health deterioration after sepsis,” said senior author McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, the Dr. Mitchell P. Fink Professor and chair of Pitt’s Department of Critical Care Medicine. “We need to focus not only on saving the patient’s life, but on ensuring the patient will have the best possible quality of life after leaving the hospital.”
Sepsis is a life-threatening organ dysfunction that can develop following an infection. It is the leading cause of death of hospital patients, and half of survivors do not completely recover, with a third of those dying in the following year and a sixth experiencing severe, persistent physical disabilities or cognitive impairment. In one study, only 43 percent of previously employed patients returned to work within a year of contracting septic shock and only 33 percent of patients living at home prior to contracting sepsis returned to living independently by six months following their discharge.
Angus and lead author Hallie Prescott, MD, MSc, assistant professor of pulmonary and critical care medicine in U-M’s Institute for Healthcare Policy & Innovation, reviewed medical literature and compiled the results of studies – with particular emphasis on clinical trials – related to sepsis survivorship.
Despite a dearth of large clinical trials to test interventions aimed at improving outcomes for sepsis patients after hospital discharge, Drs. Angus and Prescott recommend three strategies that aim to prevent long-term disabilities:
- High-quality early sepsis care that includes rapidly following protocols to help the patient fight infection, managing pain through light sedation that allows for the patient to be awakened and reoriented daily, and encouraging early mobility while the patient is still hospitalized.
- Post-discharge assessment and treatment that gets patients into rehabilitation with physical, occupational, and speech therapy shortly after discharge, and quick referral to therapists if new impairments develop.
- Screening of patients for conditions that may have been present prior to hospitalization, such as high blood pressure, and tailoring post-discharge medications to account for individual patients’ increased susceptibility to new complications. Patients and their caregivers also should be educated about sepsis and details of their hospital stay, referred to patient and caregiver support groups, and encouraged to establish their goals for future care and quality of life.
“While we are making these recommendations based on available research, many important questions about post-sepsis morbidity remain unanswered,” said Dr. Prescott. “Future research is needed to better characterize how pre-sepsis health affects long-term outcomes after sepsis so we can best tailor treatment and long-term recovery to the patient.”
Pitt Scientist Sets Sights on Gene Editing to Improve Vision

As reported by Madison Brunner for Inside UPMC, inherited retinal degeneration affects about one out of every 2,000 people worldwide and severely impacts quality of life. Due to mutated genes, this disorder causes blindness and currently has no treatment.
To solve this problem, University of Pittsburgh researchers are developing gene therapy solutions to replace the malfunctioning genes with normal, healthy genes, or to rewrite sections of mutated DNA using CRISPR/Cas9 genome editing tools.
McGowan Institute for Regenerative Medicine affiliated faculty member Leah Byrne, PhD, Assistant Professor, Department of Ophthalmology, University of Pittsburgh, is developing these gene therapy solutions with support from a competitive $300,000 Career Development Award from Research to Prevent Blindness (RPB).
One way to replace malfunctioning genes with normal ones is to ferry the good genes into the patient within harmless or inactivated viruses. The Byrne lab will use the grant to create adeno-associated viral (AAV) vectors and delivery systems for the expression of genes that don’t fit into currently available viruses, and for efficient delivery of genome editing tools in the body.
“This research will benefit millions of patients worldwide who are affected by a wide variety of forms of inherited retinal degeneration,” Dr. Byrne said. “We’re developing gene delivery methods that have the potential to impact gene therapies for any form of inherited disease.”
Since it was founded in 1960, RBP has channeled more than $355 million into eye research, and has been identified with nearly every major breakthrough in vision research.
“In my lab, we aim to directly address the most significant obstacles preventing clinical translation of gene therapies,” Dr. Byrne said. “This grant will help further our research.”
New Back Pain Study, Drug Take Back Efforts Could Lead to Decreased Opioid Use

Low back pain is the leading cause of disability worldwide and becomes increasingly common as human bodies age. Currently, more than 40 percent of low back pain patients are prescribed opioids at some point. Researchers at Pitt want to bring that number down.
“You would have to be living under a rock to not know we have an opioid crisis in the United States,” said Mike Schneider, DC, PhD, an associate professor in the University of Pittsburgh’s School of Health and Rehabilitation Sciences. “Some people are extremely susceptible and can show dependency after just a few doses. What’s sad is that some people think this crisis is just poor, uneducated people. It’s not.”
To figure out ways to decrease prescriptions and treat pain appropriately, Dr. Schneider and colleagues at Pitt are part of a five-year, $11.2 million study funded by the National Institutes of Health that will test non-drug approaches to treating and preventing chronic low back pain. Dr. Schneider, who teaches in the Department of Physical Therapy and the Clinical and Translational Science Institute, will serve as a co-principal investigator for a large multicenter clinical trial examining the effectiveness of spinal manipulation and supported self-management, compared to usual medical care. McGowan Institute for Regenerative Medicine affiliated faculty member Anthony Delitto, PhD, Professor and Dean of the School of Health and Rehabilitation Sciences at the University of Pittsburgh and Director of the Comparative Effectiveness Research Center for the University of Pittsburgh and UPMC, is also a co-principal investigator.
In Pennsylvania alone, 4,642 drug-related overdose deaths were reported in 2016 by coroners and medical examiners, an increase of 37 percent from 2015, according to a joint report by the Drug Enforcement Agency’s Philadelphia Division and the University of Pittsburgh.
Pitt’s role in the study is to be a physical therapy site, while the University of Minnesota will be a chiropractic site, and the University of Washington will assume a data management role. A total of 1,180 patients will take part in the study, with enrollment beginning next spring. Dr. Schneider said about 650 patients will be treated at the Pittsburgh site.
The researchers will compare spinal manipulation and supported self-management to usual medical care, which includes prescription medications for the care of acute lower back pain in adult patients at increased risk of becoming chronic.
The hope is that, by investigating the sources of pain, doctors can treat new episodes of back pain without prescriptions and intervene before back pain becomes a long-term problem.
“What we’re looking at is how this will affect opioid use, and that specific research question has not been looked at previously,” Dr. Schneider said. “We also want to look at whether or not we can prevent an acute episode of back pain from turning chronic.”
Non-medicinal methods of treating back pain have been gaining support in recent years.
New guidelines from the American College of Physicians state that these non-medicinal methods should be the first option to treating back pain. If medication is required for pain management, the guidelines state that non-steroidal medicines should be prescribed. Opioids are considered to be a last-resort option. The Centers for Disease Control and Prevention also published a report in 2016 acknowledging the risk of prescribing opioids to patients experiencing chronic pain.
“For the first time, we’re seeing things like spinal manipulation, yoga, tai chi, and all sorts of non-pharmacological treatments previously considered quackery or pseudoscience have evidence that show they can work,” Dr. Schneider said. “And they certainly don’t have the side effects of medication. But it’s going to take a while for the average American and American doctor to be recommending these treatments.”
SHRS and Dr. Bradley Nindl Receive DOD Project Funding

Researchers at the University of Pittsburgh School of Health and Rehabilitation Sciences (SHRS) recently received a combined $7.5 million in grant funding from the Department of Defense, Congressionally Directed Medical Research Programs. The three funded projects will study aspects of both physical and mental recovery to improve care practices for future active and retired service members. One of the projects, entitled “Studying Cognitive Readiness and Resilience,” will be co-led by McGowan Institute for Regenerative Medicine affiliated faculty member Bradley Nindl, PhD, SHRS professor and director of Pitt’s Neuromuscular Research Laboratory/Warrior Human Performance Research Center.
Given the unpredictable nature of the armed forces, military personnel face a unique set of stressors that are taxing to the mind as well as the body. Dr. Nindl and Anne Germain, PhD, associate professor of psychiatry at the Pitt School of Medicine and director of Pitt’s Sleep and Behavioral Neuroscience Center, received $2.5 million to study the mental readiness and resiliency of military service members. This grant will be used to develop a comprehensive series of cognitive, behavioral, and sensorimotor readiness metrics to evaluate, validate, and encourage mental preparedness for military service.
The other two projects funded include “Determining Optimal Timing of Surgery and Rehabilitation for Knee Injuries” and “Avoiding Complications When Using Prosthetics.”
“We are honored to use our expertise to advance health care and quality of life for those who have so valiantly served our country,” said McGowan Institute affiliated faculty member David Brienza, PhD, SHRS associate dean of research. “These three projects fill critical research needs and stand to make a tremendous difference in the lives of our country’s military personnel.”
Illustration: SHRS.
AWARDS AND RECOGNITION
Dr. Robert Parker Receives 2017 Pommersheim Award for Excellence in Teaching

Recognizing his significant accomplishments over the past year, McGowan Institute for Regenerative Medicine affiliated faculty member Robert Parker, PhD, Full Professor, was the 2017 winner of the James Pommersheim Award for Excellence in Teaching in Chemical Engineering at the annual Department of Chemical and Petroleum Engineering gathering.
In presenting the award, Department Chair and McGowan Institute faculty member Steven Little, PhD, William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology, noted that Dr. Parker was promoted to Vice Chair for Graduate Education, graduated four PhD students (in one year), published eight papers, ran an REU program, received a 4.75 OTE score in 0500 Process Systems with 63 students (as an overload teaching assignment), established ENGR 1933 Engineering a Craft Brewery with the most successful first-offering elective in the history of the Swanson School, and won the Swanson School of Engineering’s Outstanding Educator Award this fall.
The Pommersheim Award was established by the Department and James M. Pommersheim (’70) to recognize departmental faculty in the areas of lecturing, teaching, research methodology, and research mentorship of students. Dr. Pommersheim, formerly Professor of Chemical Engineering at Bucknell University, received his bachelor’s, master’s, and PhD in chemical engineering from Pitt.
Congratulations, Dr. Parker!
Illustration: Dr. Pommersheim (left) with Dr. Parker. University of Pittsburgh Swanson School of Engineering.
Dr. Rory Cooper Named a Health Hero by O, the Oprah Magazine

McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, has been named a Health Hero by O, The Oprah Magazine. Dr. Cooper—the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, and also the Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt—was one of 14 “changemakers” featured in the magazine’s January 2018 issue.
Dr. Cooper was lauded for his work designing devices “with independence and dignity in mind.”
Congratulations, Dr. Cooper!
Illustration: O, the Oprah Magazine.
Bridgeside Research Forum Fall 2017 Travel Award Winners Announced

The Bridgeside Research Forum is a seminar given by postdocs and graduate students every Friday during the University of Pittsburgh academic year.
The Bridgeside Research Forum Fall 2017 Travel Awards for best presentation were recently announced and the winners are as follows (left the right):
- Eoghan Cunnane, PhD (Lab of David Vorp, PhD, Swanson School of Engineering)
- Megan Routzong (Abramowitch Lab, Swanson School of Engineering)
- Lance Thurlow, PhD (Richardson Lab, Department of Microbiology and Molecular Genetics)
- Catherine Go, PhD (Lab of Bryan Tillman, MD, PhD, McGowan Institute for Regenerative Medicine, received by Jenna Kuhn)
- Juraj Adamik, PhD (Lab of Deborah Galson, PhD, Department of Microbiology and Molecular Genetics)
- Giovanna Francipane, PhD (Lab of Eric Lagasse, PharmD, PhD, McGowan Institute for Regenerative Medicine)
Congratulations, all!
Joseph Bartolacci, Badylak Lab Student, Receives George Bevier Fellowship in Engineering
Joseph Bartolacci is an MD candidate from Temple University School of Medicine in Philadelphia who spends summers in the laboratory of McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the Institute.
In the lab, Mr. Bartolacci’s research focuses on exploring the effects of extracellular matrix (ECM) degradation products on macrophage phenotypes. He is also involved in projects related to the effects of exosomes in ECM. He has co-authored two manuscripts and presented his work at two conferences.
The George M. Bevier Fellowship in Engineering supports programs in bioengineering, sustainability, and energy and energy resources.
Congratulations, Mr. Bartolacci!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#179 –– Dr. Fatima Syed-Picard is an Assistant Professor in the Department of Oral Biology, University of Pittsburgh, School of Dental Medicine. She is also a faculty member in the Center for Craniofacial Regeneration. Dr. Syed-Picard discusses her research on craniofacial tissue engineering.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Xiao X, Guo P, Shiota C, Zhang T, Coudriet GM, Fischbach S, Prasadan K, Fusco J, Ramachandran S, Witkowski P, Piganelli JD, Gittes GK.
Title: Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes
Summary: Successful strategies for treating type 1 diabetes need to restore the function of pancreatic beta cells that are destroyed by the immune system and overcome further destruction of insulin-producing cells. Here, we infused adeno-associated virus carrying Pdx1 and MafA expression cassettes through the pancreatic duct to reprogram alpha cells into functional beta cells and normalized blood glucose in both beta cell-toxin-induced diabetic mice and in autoimmune non-obese diabetic (NOD) mice. The euglycemia in toxin-induced diabetic mice and new insulin+ cells persisted in the autoimmune NOD mice for 4 months prior to reestablishment of autoimmune diabetes. This gene therapy strategy also induced alpha to beta cell conversion in toxin-treated human islets, which restored blood glucose levels in NOD/SCID mice upon transplantation. Hence, this strategy could represent a new therapeutic approach, perhaps complemented by immunosuppression, to bolster endogenous insulin production. Our study thus provides a potential basis for further investigation in human type 1 diabetes.
Source: Cell Stem Cell. 2018 Jan 4;22(1):78-90.e4.
GRANT OF THE MONTH
PI: Mike Schneider, DC, PhD
Co-PI: Anthony Delitto, PhD
Description: The researchers will compare spinal manipulation and supported self-management to usual medical care, which includes prescription medications for the care of acute lower back pain in adult patients at increased risk of becoming chronic.
Source: National Institutes of Health
Term: 5 years
Amount: $11.2 million
