January 2016 | VOL. 15, NO. 1| www.McGowan.pitt.edu
Grant Received for the Alliance for Regenerative Rehabilitation Research & Training
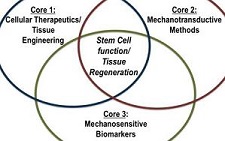
McGowan Institute for Regenerative Medicine affiliated faculty members Michael Boninger, MD, UPMC Endowed Chair and Professor with Tenure in the Department of Physical Medicine & Rehabilitation, University of Pittsburgh, and Thomas Rando, MD, PhD, Professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, are the principal investigators of the recently awarded $1,124,000, 5-year grant from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development. The overarching goal of the project entitled, “Alliance for Regenerative Rehabilitation Research & Training (AR3T),” is to establish a national network that will expand scientific knowledge, expertise, and methodologies across the domains of regenerative medicine and rehabilitation.
The project team includes a multidisciplinary group of investigators that brings leadership and vision across the domains of rehabilitation and regenerative medicine. Dr. Boninger has long focused on the integration of innovative technologies into rehabilitation practice, as evidenced by his pioneering work in robotics, bioengineering, and neuroprosthetics. Dr. Rando’s seminal basic science investigations have transformed our understanding of the basic biology of stem cells and tissue regeneration. McGowan Institute for Regenerative Medicine affiliated faculty member Fabrisia Ambrosio, PhD, MPT, Assistant Professor in the Department of Physical Medicine & Rehabilitation, University of Pittsburgh, Linda Noble-Haeusslein, PhD (University of California, San Francisco), Carmen Terzic, MD, PhD (Mayo Clinic), and Gwendolyn Sowa, MD, PhD (University of Pittsburgh), will serve as co-investigators, offering complementary expertise and leadership in musculoskeletal, neurological, and cardiovascular research. Together, these five investigators have a strong track record of successful collaborative efforts dedicated to advancing the field of Regenerative Rehabilitation.
The advancement of regenerative medicine principles and technologies holds great potential to drive progress in the prevention and treatment of individuals with a host of pathologies resulting from injury, disease, or aging. The long-term goal of regenerative medicine is to promote the repair, replacement, or regeneration of tissues. Likewise, rehabilitation seeks to harness the body’s innate regenerative potential in order to maximize function. Both fields hold great potential to drive progress in the treatment of a host of acute and chronic pathologies. The team proposes that these two fields are inextricably intertwined; an intersection of disciplines known as Regenerative Rehabilitation. To fully realize the tremendous potential of Regenerative Rehabilitation, promoting the interaction of basic scientists with rehabilitation specialists must be realized. Rehabilitation clinicians who can help oversee the quality, safety, and validity of these innovative Regenerative Rehabilitation technologies must also be trained.
Toward this end, AR3T will provide didactic training that exposes rehabilitation researchers to cutting-edge investigations and state-of-the-art technologies in the field of regenerative medicine. AR3T will drive the science underlying Regenerative Rehabilitation by:
- cultivating collaborative opportunities among rehabilitation researchers and internationally renowned investigators in the field of regenerative medicine
- launching a pilot funding program to support novel lines of Regenerative Rehabilitation investigations
- developing and validating technologies to elucidate the stem cell response to extrinsic mechanical signals
Illustration: Alliance for Regenerative Rehabilitation Research & Training (AR3T).
RESOURCES AT THE MCGOWAN INSTITUTE
February Special at the Histology Lab
Don’t let the Winter Blues get you down! Get down with the winter Blues! Visit the McGowan Institute Histology Lab and make the most of Blue moods.
Shake off the winter blues and get down with 20% off these Special Stain Blues, when you mention this ad. Contact Lori, in Histology with any questions regarding our February Blue Specials or any of your histology needs. Most projects can be completed and returned to you in one week. The McGowan Histology Lab offers free pick-up and delivery to Oakland. Email perezl@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample?
Contact Lori to find out how!
Utilizing Fluorescence-Activated Cell Sorting at the Flow Cytometry Facility
Kyle Orwig, PhD, is a Professor of Obstetrics, Gynecology and Reproductive Sciences and Developmental Biology in the University of Pittsburgh School of Medicine and maintains his laboratory in Magee-Womens Research Institute. He is the Director of the Molecular Genetics and Developmental Biology Graduate Program and founding Director of the Fertility Preservation Program in Pittsburgh. Dr. Orwig uses the Flow Cytometry Facility for many of his research projects.
Chemotherapy and radiation treatments for cancer can cause permanent infertility. The Orwig Lab is actively studying the potential of stem cells to preserve the fertility of cancer patients and restore the fertility of cancer survivors. In a nonhuman primate model of cancer survivorship, his lab was the first to demonstrate that spermatogonial stem cells (SSCs) from the testis can be obtained by biopsy and frozen prior to therapy. They can then be thawed at a later date, enriched by fluorescence-activated cell sorting (FACS), and transplanted into the testes to regenerate spermatogenesis in monkeys that were rendered infertile by chemotherapy treatment. Sperm from the transplanted stem cells were competent to fertilize monkey eggs and give rise to preimplantation embryo development. Dr. Orwig recently completed an R01 grant (HD055475) on this topic that used the McGowan Institute for Regenerative Medicine Flow Cytometry Facility extensively and resulted in several publications.
Anticipating that SSCs can be used to preserve and restore the fertility of human cancer patients, many centers in the US and abroad are actively freezing testicular tissues for patients, anticipating that stem cell therapies will be available in the future to help survivors achieve their fertility goals. There is a concern, however, that cryopreserved testicular cells obtained from a cancer patient before he starts therapy could be contaminated with malignant cells. Therefore, it is necessary to devise sorting strategies to isolate and enrich therapeutic stem cells while removing malignant contamination.
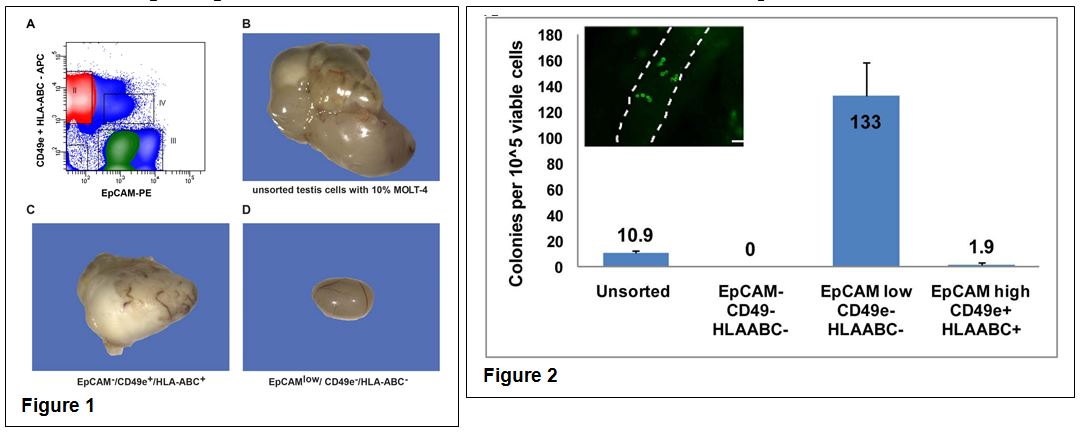
In two separate studies, Dr. Orwig’s group demonstrated that it was possible to remove leukemic contamination from both monkey and human testicular cells using the capabilities of the Flow Cytometry Facility. Antibodies for numerous cell surface markers were screened to identify markers that were expressed by leukemic cells, but not spermatogonia and also markers that were expressed by spermatogonia, but not leukemic cells. The screening revealed that MOLT-4 leukemia cells expressed CD49e and HLA-ABC, but not Ep-CAM. In contrast, human spermatogonia expressed Ep-CAM, but not CD49e or HLA-ABC.
To determine whether a positive/negative sorting strategy using these markers could be used to effectively isolate and enrich therapeutic SSCs, while removing malignant contamination, a suspension of human testis cells was contaminated with MOLT-4 leukemia cells. The heterogeneous population was then fractionated and sorted based on expression of CD49e, HLA-ABC and EpCAM (Fig. 1A). While the unsorted cell suspension and the Ep-CAM–/CD49e+/HLA-ABC+ fraction produced tumors when transplanted into mouse testes (Fig. 1B and 1C), no tumors were produced from the Ep-CAMlow/CD49e–/HLA-ABC– fraction (Fig. 1D). Human to nude mouse xenotransplantation was used to confirm stem cell activity in the Ep-CAMlow/CD49e–/HLA-ABC– fraction. The transplant data revealed stem cell activity was enriched more than 12-fold in the Ep-CAMlow/CD49e–/HLA-ABC– fraction compared to unsorted cells (Fig 2.), indicating that it is possible to isolate and enrich therapeutic human spermatogonia while removing malignant cells from a contaminated human testis cell suspension.
The Flow Cytometry Facility at the McGowan Institute is essential to many aspect of research in the Orwig Lab and most importantly is run by a terrific operator, Lynda Guzik. Dr. Orwig is currently funded by P01HD075795 and R01HD076412. Both grants employ FACS extensively to isolate and enrich stem cells prior to transplantation and to facilitate their molecular characterization.
Contact Lynda Guzik, the Flow Cytometry Facility Manager, for more information on how the Facility’s resources can help you: 412-648-8660 or guzilj@upmc.edu.
UPCOMING EVENTS
Registration Open: 2016 McGowan Institute Retreat
 The 15th Annual McGowan Institute for Regenerative Medicine Scientific Retreat is set to take place on March 6-8, 2016, at Nemacolin Woodlands Resort.
The 15th Annual McGowan Institute for Regenerative Medicine Scientific Retreat is set to take place on March 6-8, 2016, at Nemacolin Woodlands Resort.
The retreat will begin on Sunday afternoon- March 6, 2016- with a series of short courses, followed by a poster session, informal mixer, and keynote session.
Dr. Fabrisia Ambrosio and Dr. Vijay Gorantla lead the 2016 program committee that has planned an exciting mix of speakers and topics.
Highlights from the 2015 Retreat can be found here.
Registration information for the 2016 Retreat is available here.
Save the Date: McGowan Institute Distinguished Lecture

The McGowan Institute’s Spring Distinguished Lecture Series has been announced. The Distinguished Lecturer is Robert H. Bartlett, MD, Emeritus Professor of Surgery, University of Michigan. He will speak on “ECLS, Past, Present, and Future.”
The lecture is scheduled for Thursday, March 31, 2016 at 4:00 pm. It will be held at Scaife Hall Auditorium 5. A reception will follow at 5:00 pm in Scaife Hall Room 1105A.
Save the Date: 9th Symposium on Biologic Scaffolds for Regenerative Medicine
The 9th Symposium on Biologic Scaffolds for Regenerative Medicine will be held at the Silverado Resort in Napa, California on April 28th – 30th, 2016.

Although all topic areas are considered and will be represented, the 2016 symposium will definitely include the following:
- An in depth account of the most recent findings regarding the mechanisms by which biologic scaffolds facilitate constructive remodeling of tissues; including body wall (skeletal muscle), cardiovascular, reconstructive surgical applications such as breast and pelvic floor, and whole organs such as liver, lung and heart.
- Identification and discussion of manufacturing issues such as tissue source, decellularization methods, and sterilization decisions that affect the quality and performance of biologic scaffolds for surgical applications.
- A review of clinical experiences, especially general surgery, orthopedic and trauma related challenges, neurologic applications, gastrointestinal applications, and cardiovascular applications.
- Identification and discussion of the effect of the host innate immune response upon scaffold remodeling and clinical outcome.
List of invited (and accepted) speakers to date: Robert M. Nerem, PhD (Georgia Institute of Technology), Arnold I. Caplan, PhD (Case Western Reserve University), Jeffrey M. Davidson, Ph.D. (Vanderbilt University), Cyrus Ghajar, PhD (Fred Hutchinson Cancer Research Center), Jeffrey A. Hubbell, PhD (Swiss Federal Institute of Technology, EPFL), Kristen Jones, MD (University of Minnesota), C. James Kirkpatrick MD PhD DSc FRCPath (Johannes Gutenberg University), Robert G. Martindale, MD, PhD (Oregon Health & Science University), Charles D. Mills, PhD (BioMedical Consultants), Laura E Niklason PhD, MD (Yale University), Frederick J. Schoen, MD, PhD (Harvard University), Allan S. Stewart, MD (Mount Sinai Hospital NYC), and Nadia Rosenthal, PhD (Monash University, Australia).
SCIENTIFIC ADVANCES
Clinical Milestone Reached with 500 Minimally Invasive Heart Valve Repairs

A team of surgeons and interventional cardiologists at the University of Pittsburgh Medical Center (UPMC) recently performed their 500th transcatheter aortic valve replacement (TAVR), more than any other heart program in the region. The minimally invasive procedure repairs the aortic valve in patients with severe aortic stenosis, a debilitating narrowing of the heart’s aortic valve that causes shortness of breath, lightheadedness, and fatigue.
“TAVR does not require opening the heart, and we can avoid placing the patient on a heart-lung machine,” said McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gleason, MD, chief of the division of cardiac surgery at UPMC. “As the largest heart surgery program in Pennsylvania, the UPMC Heart and Vascular Institute has access to more of the new transcatheter valve technologies than any other program in the region, and we are continuing to evaluate new designs for these life-saving devices.” Dr. Gleason as serves as an Associate Professor of Surgery, Division of Cardiothoracic Surgery, University of Pittsburgh School of Medicine, with a secondary appointment in Pitt’s Department of Bioengineering, Swanson School of Engineering.
UPMC physicians began performing the TAVR procedure in 2011 and now are among the most experienced in the country.
The treatment greatly improves the quality of life for those who suffer from aortic stenosis, which affects as many as 500,000 people in the U.S. This condition can interfere with daily activities, such as walking or climbing stairs. Previously, a patient’s only option was to have open-heart surgery to replace the aortic valve, but the procedure often was deemed too risky for elderly patients, who are most prone to the condition.
TAVR involves implanting an artificial valve through a catheter, or thin tube, inserted through a large artery in the leg or chest. As a leading center, UPMC is the only program in the region to have four different types of catheter valves available for implantation, two of which are FDA-approved and two of which are available only through clinical trials in the U.S.
“Open heart surgery is not the ideal option for every patient,” said John Schindler, MD, co-director of the Center for Aortic Valve Disease at UPMC. “TAVR provides an additional aortic valve replacement option for high-risk patients who would benefit from a less-invasive procedure.”
Working in Collaboration to Prevent Blindness

As reported by Heather Chronis in the Fall 2015 Sight & Sound newsletter of the Eye and Ear Foundation of Pittsburgh, the Ophthalmology and Visual Sciences Research Center at the University of Pittsburgh, under the direction of McGowan Institute for Regenerative Medicine affiliated faculty member Robert Hendricks, PhD, Joseph F. Novak Professor and Vice-Chair for Research in the Department of Ophthalmology at the University of Pittsburgh School of Medicine, has been the recipient of multiple restricted and unrestricted grants from the Research to Prevent Blindness (RPB). Founded in 1960 by Dr. Jules Stein, RPB is a national leader in the effort to fund, coordinate, and promote vision research in the United States. From its infancy to today, RPB has advocated at all levels for vision research. RPB restricted and unrestricted grants have helped to launch the careers of countless vision scientists.
Indeed, many leading vision scientists around the world credit the RPB with their early scientific development. The unrestricted grants to the University of Pittsburgh have supported pilot projects including recent projects by McGowan Institute for Regenerative Medicine affiliated faculty members James Funderburgh, PhD, Professor of Ophthalmology at the University of Pittsburgh and the Associate Director of the Louis J. Fox Center for Vision Restoration, and Yiqin Du, MD, PhD, Assistant Professor in the Departments of Ophthalmology and Developmental Biology at the University of Pittsburgh School of Medicine and the Fox Center, to study the use of corneal stromal stem cells to treat corneal scarring, by Dr. Du to study the use of trabecular meshwork stem cells to treat glaucoma, by McGowan Institute for Regenerative Medicine affiliated faculty member Shiva Swamynathan, PhD, Associate Professor with the Department of Ophthalmology in the University of Pittsburgh School of Medicine, to study the anti-inflammatory role of SLURP-1, and by McGowan Institute for Regenerative Medicine affiliated faculty member Ian Sigal, PhD, Assistant Professor in the Laboratory of Ocular Biomechanics in the Department of Ophthalmology at the University of Pittsburgh School of Medicine, to study ocular biomechanics in glaucoma. All of these studies are now funded by National Institute of Health (NIH) R01 grants. Work by McGowan Institute for Regenerative Medicine affiliated faculty members Kevin Chan, PhD, Assistant Professor in the Departments of Ophthalmology and Bioengineering at the University of Pittsburgh, and Ian Conner, MD, PhD, Assistant Professor of Ophthalmology and Bioengineering at the University of Pittsburgh, investigating the structural, metabolic, and functional relationships between the eye and the brain in glaucoma was also funded by an RPB unrestricted grant and currently has an NIH R01 application pending. In addition to funding pilot projects, RPB unrestricted grants have also helped with physical improvements to the research labs. One of the most valuable uses of the RPB funding is the purchase of lab equipment for new faculty. Additionally, service contracts on core equipment have been funded, which has helped to preserve equipment obtained through other grants.
The research faculty of the University of Pittsburgh has also received many individual grants from RPB. Dr. Funderburgh received a Stein Professorship, a $625,000 award for a researcher who is moving from a basic science department to an ophthalmology department. This grant helped Dr. Funderburgh move his laboratory from University of Kansas to the University of Pittsburgh. Xiangyun Wei, PhD, Robert Shanks, PhD, and McGowan Institute for Regenerative Medicine affiliated faculty member Matthew Smith, PhD, Assistant Professor in the Department of Ophthalmology, University of Pittsburgh, have all received Career Development Awards, which include a $200-250,000 award to promote their early vision research career development. Drs. Hendricks and Michael Gorin were awarded the Senior Scientific Investigator Award designed to support high risk/high reward projects of senior investigators, Sherrie Divito, MD, Jared Knickelbein, MD, and Daniel Roh, MD, PhD, all won the RPB Medical Student Award, Dr. Wei won the Wasserman Award, while George Stetten, MD, PhD, and McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering, University of Pittsburgh, each won the Innovative Ophthalmic Research Award. Over the last 20 years, the total amount of RPB individual grants to the Ophthalmology Department have totaled $1,690,000, providing the researchers with the opportunity to continue their study of unique and targeted approaches to prevent vision loss. RPB support to the Ophthalmology Department has increased each year, which has allowed the institution to grow in stature in the ophthalmology research arena since Dr. Hendricks’ arrival in 1997. From this groundbreaking work, has come NIH grants which are considered the most prestigious in the United States and are considered a predictor of great future successes. The funding by the RPB has provided the groundwork for international success for the Ophthalmology Department at the University of Pittsburgh.
Illustration: The Louis J. Fox Center for Vision Restoration.
Unraveling the Genetics of Pregnancy and Heart Failure

Scientists including McGowan Institute for Regenerative Medicine affiliated faculty member Dennis McNamara, MD, Professor of Medicine, University of Pittsburgh, and Director of the Heart Failure/Transplantation Program at the University of Pittsburgh Medical Center, have found that women who suffer unexplained heart failure towards the end of pregnancy or shortly after giving birth share certain genetic changes.
The finding provides some explanation for this mysterious condition, and suggests that by testing relatives, other women who carry the same genes, and who might face similar risks, could be identified early. They could then be monitored closely and treated more swiftly if needed. In the future, preventative treatment might be developed too.
Normal pregnancy is a challenge for the cardiovascular system, with up to a 25 per cent increase in heart rate, and 50 per cent increase in the volume of blood pumped in a minute. In some previously healthy women the heart just can’t cope. Around the time of childbirth the heart enlarges, and stops pumping properly – classic symptoms of heart failure. This can lead to death or the need for a heart transplant, and at the moment doctors don’t know who this will happen to.
Around 1 in every 1000 women in Europe and the US suffers from this condition, known as peripartum cardiomyopathy (PPCM). Elsewhere there are geographic hotspots, including Nigeria and Haiti, where the number can be as high as 1 in 100.
Women who suffer from pre-eclampsia, those pregnant with twins, and older pregnant women are all known to be at higher risk of developing PPCM. The cause is unknown, though theories about contributing factors include: an auto-immune response, undiagnosed heart damage, too much salt or too little selenium in the diet.
The teams found that the 170 or so women they tested carried a higher number of genetic changes than normal. The team decoded genes that can cause rare inherited forms of cardiomyopathy, and found that women with PPCM had a very similar genetic profile to patients with dilated cardiomyopathy (DCM) – a condition that runs in families.
Cardiologist James Ware, Clinical Senior Lecturer in Genomic Medicine at the Medical Research Council’s (MRC’s) Clinical Sciences Centre (CSC), based at Imperial College, is lead author of the paper describing the results, which he described as encouraging news: “DCM is managed as an inherited condition: first degree relatives of affected individuals are offered genetic screening. Our results suggest that genetic diagnostics and family management may have similar value in PPCM.”
“PPCM has a mortality rate of 5 to 10 per cent, so being able to shed light on why it occurs in some women and not others is an important development and could ultimately save lives. Further research is needed to better understand the value of genetic information in determining the prognosis of PPCM.”
The study follows collaboration between researchers at a number of centers around the world including the Royal Brompton Hospital in London, Harvard Medical School, the Perelman School of Medicine at the University of Pennsylvania, and the University of Pittsburgh Medical Center at the University of Pittsburgh in the US.
The researchers found that women with PPCM and patients with DCM shared abnormalities in a gene that codes for a protein called titin. Titin is the largest protein in the human body and acts like a spring within muscle tissue, including the heart. Titin’s size is crucial to its function, and some DCM is due to mutations that disrupt titin and make the protein shorter. This leads to hearts that are ‘baggy’ and stretched with thin muscle walls, that are too weak to pump blood around the body effectively, and which can cause sudden death in the 1 in 250 people affected.
The researchers found that in women with this genetic variant their heart was pumping less well after a year. Testing for this change may therefore prove a useful way to predict likely outcome, enabling closer surveillance of those at higher risk.
This latest study was led by Dr. Zoltan Arany in the Perelman School of Medicine at the University of Pennsylvania together with professors Christine and Jon Seidman and Dr. McNamara at the University of Pittsburgh Medical Center. Co-authors include Dr. Stuart Cook, who leads the Cardiovascular Magnetic Resonance Imaging and Genetics group at the CSC; Dr. Sanjay Prasad, a consultant cardiologist and honorary senior lecturer at Imperial College; and Francesco Mazzarotto, a PhD student working with Dr. Cook and Dr. Ware at the National Heart and Lung Institute.
The study builds on a substantial program of research around the role of titin in the heart by the teams from the CSC, Imperial College, and Harvard.
Sedentary Behavior Linked to Poor Health in Adults with Severe Obesity, Independent of Exercise

Sedentary behavior is associated with poor cardiovascular health and diabetes in adults with severe obesity, independent of how much exercise they perform, a University of Pittsburgh Graduate School of Public Health-led study showed for the first time. McGowan Institute for Regenerative Medicine affiliated faculty member Steven Belle, PhD, MScHyg., professor in the Department of Epidemiology, Graduate School of Public Health at the University of Pittsburgh, and a co-director in the Epidemiology Data Center in the Graduate School of Public Health, is a co-author of the study.
The finding could be used to design and test programs for adults with severe obesity that emphasize reducing time spent sitting, rather than immediately working toward increased moderate- to vigorous-intensity physical activity or exercise, such as brisk walking. In the U.S., 15 percent of adults have severe obesity, placing them at high risk of cardiovascular and metabolic disease, and premature mortality.
“Adults with severe obesity often have difficultly following national guidelines to participate in at least 30 minutes per day of moderate- to vigorous-intensity physical activity for health benefits,” said lead author Wendy C. King, PhD, associate professor in the Department of Epidemiology at Pitt Public Health. “Our findings suggest that replacing sedentary behavior, like watching television or sitting at the computer, with low-intensity physical activities, such as light housework or going for a casual stroll, may improve cardiometabolic health in this population.”
In addition, Dr. King and her colleagues determined that defining “sedentary time” as 10 minutes or more without walking yielded stronger associations between sedentary behavior and cardiometabolic health compared to allowing sedentary time to be as short as one minute, which has been the norm in the field.
“This is important because accurate assessment of sedentary behavior is crucial to being able to evaluate if and how this behavior is related to health outcomes. If our estimate of sedentary behavior is poor, we may not detect true associations,” said Dr. King.
She and her colleagues followed 927 patients participating in the Longitudinal Assessment of Bariatric Surgery-2, a prospective study of patients undergoing weight-loss surgery at 1 of 10 different hospitals across the U.S. For a 1-week period before surgery, the research team measured the participants’ activity—or lack of activity—using monitors that tracked the number of steps taken each minute.
For every hour per day participants spent in sedentary bouts of at least 10 minutes, their odds of having diabetes increased by 15 percent, metabolic syndrome by 12 percent, and elevated blood pressure by 14 percent, and their waist circumference was a half inch larger, after adjusting for their sex, age, household income, smoking status, alcohol use, depressive symptoms, body mass index (BMI), and time spent in moderate- to vigorous-intensity physical activity.
Future research is needed to determine whether replacing sedentary behavior with low-intensity physical activity is an effective approach to preventing and managing cardiovascular and metabolic diseases in adults with severe obesity, and evaluate strategies to help this population make such lifestyle changes.
Additional investigators on this research are Jia-Yuh Chen, MS, and Anita P. Courcoulas, MD, MPH, of Pitt; James E. Mitchell, MD, and Brian Cook, PhD, both of the Neuropsychiatric Research Institute; Bruce M. Wolfe, MD, of the Oregon Health & Science University; Emma J. Patterson, MD, of the Legacy Good Samaritan Weight Management Institute in Portland, Ore.; William B. Inabnet, MD, of Mount Sinai Hospital in New York; Gregory F. Dakin, MD, of Weill Cornell Medical College; David R. Flum, MD, MPH, of the University of Washington.
Strengthening a Tiny Heart, Pound for Pound
As reported by Heather Boerner of PittMed, the heart of a newborn is small but powerful—usually. For babies born with heart defects, though, the muscle can be marred by holes and malformed valves, or simply pump weakly. Surgery can close the holes and fix the valves.
But a weak heart? Medicine hasn’t been able to do much about that. Beta blockers and ACE inhibitors, common treatments for adults, are ineffective in children. Worse, corrective surgeries can cause scar tissue that further limits the heart’s power to pump. Left untreated, a weak heart can cause pediatric heart failure.
“This is where regeneration comes in,” says McGowan Institute for Regenerative Medicine affiliated faculty member Bernhard Kühn, an MD associate professor of pediatrics at Pitt and director of research in the Division of Pediatric Cardiology at Children’s Hospital of Pittsburgh of UPMC. “If we can give these kids just half an ounce [more] heart muscle, that may potentially make a big difference for the duration and quality of their lives.”
And it seems Dr. Kühn might have found a way to do that, at least in the lab. As reported in a Science Translational Medicine paper published in April, Dr. Kühn’s team applied to injured mouse hearts and isolated human tissue a protein called recombinant growth factor neuregulin-1 (rNRG1). The protein is known to stimulate cell growth in many organs and the nervous system. Dr. Kühn’s team stimulated cell growth. This complex, multipart study not only found rNRG1 to be effective but also pinpointed the narrow window in which to target future clinical trials.
In a previous study, Dr. Kühn and his team found that people with healthy hearts can generate new heart cells until about age 20. But no one knew how long an infant with an unhealthy heart would be able to grow new heart cells. So they set out to answer that question, as well as the question of whether rNRG1 could kick-start the process.
After fine-tuning their own adaptation of an animal model of pediatric heart disease, the team studied the timing of rNRG1 administration. They had assumed starting at 4 days old would be plenty early—but not so. Frustrated, they started all over again, introducing the protein at birth.
Everything changed. “When we started seeing positive results,” says Balakrishnan Ganapathy, a lab manager and research technologist at Children’s, “we realized we were on to something really big.”
Among rodents that received rNRG1 at birth, only one in 10 still had a scar across the entire thickness of the heart wall when they were examined later (compared to 75 percent of the hearts that received rNRG1 at 4 days old). That is to say, rNRG1 seems to generate cell growth, which can repair heart damage and build a stronger muscle. What’s more, rNRG1 seems to protect healthy heart tissue from dying.
“We do now have, in the data, some indication that the scar is now pumping [blood],” says Dr. Kühn.
At the same time, the lab was studying human heart tissue from infants who’d undergone heart surgery. They found that in the dish, the heart cells stopped proliferating in patients older than 6 months of age. And for those infants who did exhibit cell growth, it was slow.
But when they applied rNRG1 to the cells? “The surprising thing is that the heart-muscle pieces actually liked it,” says Dr. Kühn. “They had striations, so their molecular motors were visible with the microscope. The striations were still parallel, meaning when they contracted, they all pulled in synchronicity. The electrical connections were still present.”
Dr. Kühn and his team are hopeful that the same treatment might be effective in clinical trials that target children from birth to 6 months of age. Mr. Ganapathy, meanwhile, has begun studying the safety of rNRG1 administration in rodents. So far, he’s seen no serious side effects.
In this era of ever-improving neonatal medicine and surgery, the question is less often, Can we save this baby? says Dr. Kühn. “The question is,” he says, “How will this person live when he or she is 10, 20, 30, 40, 50, 60, 70, even 80 years old?”
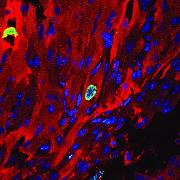
Illustration: A Pitt lab may have found a way to strengthen and heal cardiac tissue in infants born with weak hearts. Here we see a cardiomyocyte (red) undergoing cellular proliferation (green). Blue spots are cell nuclei. –Balakrishnan Ganapathy.
Hybrid Material that Responds to Heat and Light Presents Future Potential for 4D-Printed Adaptive Devices
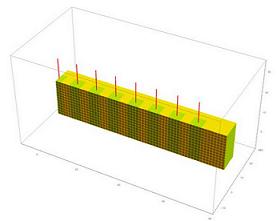 Combining photo-responsive fibers with thermo-responsive gels, researchers at the University of Pittsburgh’s Swanson School of Engineering and Clemson University have modeled a new hybrid material that could reconfigure itself multiple times into different shapes when exposed to light and heat, allowing for the creation of devices that not only adapt to their environment, but also display distinctly different behavior in the presence of different stimuli.
Combining photo-responsive fibers with thermo-responsive gels, researchers at the University of Pittsburgh’s Swanson School of Engineering and Clemson University have modeled a new hybrid material that could reconfigure itself multiple times into different shapes when exposed to light and heat, allowing for the creation of devices that not only adapt to their environment, but also display distinctly different behavior in the presence of different stimuli.
Computational modeling developed by McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering at Pitt, and Olga Kuksenok, PhD, Associate Professor of Materials Science and Engineering at Clemson’s College of Engineering and Science, predicted these composites would be both highly reconfigurable and mechanically strong, signaling a potential for biomimetic four-dimensional printing. Their research, “Stimuli-responsive behavior of composites integrating thermo-responsive gels with photoresponsive fibers,” was recently published in the journal Materials Horizons, published by the Royal Society of Chemistry.
“In 4D printing, time is the fourth dimension that characterizes the structure of the material; namely, these materials can change shape even after they have been printed. The ability of a material to morph into a new shape alleviates the need to build a new part for every new application, and hence, can lead to significant cost savings,” Dr. Balazs explained. “The challenge that researchers have faced is creating a material that is both strong and malleable and displays different behavior when exposed to more than one stimulus.”
Drs. Balazs and Kuksenok resolved this issue by embedding light-responsive fibers, which are coated with spirobenzopyran (SP) chromophores, into a temperature-sensitive gel. This new material displays distinctly different behavior in the presence of light and heat.
“If we anchor a sample of the composite to a surface, it will bend in one direction when exposed to light, and in the other direction when exposed to heat,” Dr. Kuksenok said. “When the sample is detached, it shrinks like an accordion when heated and curls like a caterpillar when illuminated. This programmable behavior allows a single object to display different shapes and hence functions, depending on how it is exposed to light or heat.”
The researchers note that by localizing the SP functionality specifically on the fibers, the composites can encompass “hidden” patterns that are only uncovered in the presence of light, allowing the material to be tailored in ways that would not be possible by simply heating the sample. This biomimetic, stimuli-responsive motion could allow for joints that bend and unbend with light and become an essential component for new adaptive devices, such as flexible robots.
“Robots are wonderful tools, but when you need something to examine a delicate structure, such as inside the human body, you want a “squishy” robot rather than the typical devices we think of with interlocking gears and sharp edges,” Dr. Balazs said. “This composite material could pave the way for soft, reconfigurable devices that display programmed functions when exposed to different environmental cues.”
As Dr. Balazs points out, “the real significance of the work is that we designed a single composite that yields access to a range of dynamic responses and structures. On a conceptual level, our results provide guidelines for combining different types of stimuli-responsive components to create adaptive materials that can be controllably and repeatedly actuated to display new dynamic behavior and large-scale motion.”
Future research with this discovery will focus on tailoring the arrangements of the partially-embedded fibers to create hand-like structures that could serve as a type of gripper.
Illustration: University of Pittsburgh Swanson School of Engineering.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#155 –– Michael Boninger is the UPMC Endowed Chair and Professor in the Department of Physical Medicine & Rehabilitation, and the Senior Associate Medical Director, Human Engineering Research Laboratories, University of Pittsburgh. He is also the Director of the UPMC Rehabilitation Institute. Dr. Boninger discusses recent developments in regenerative rehabilitation.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
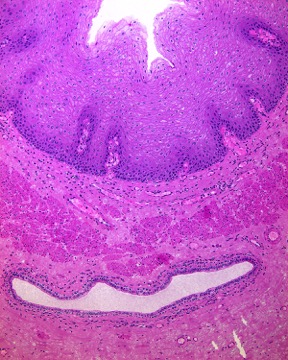
Image of a normal, human esophagus, H&E stained.
Image by Donna Beer Stolz, PhD
PUBLICATION OF THE MONTH
Author: Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley-Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J 3rd, McNamara DM, Seidman CE, Seidman JG, Arany Z; IMAC-2 and IPAC Investigators
Title: Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies
Summary: Background Peripartum cardiomyopathy shares some clinical features with idiopathic dilated cardiomyopathy, a disorder caused by mutations in more than 40 genes, including TTN, which encodes the sarcomere protein titin. Methods In 172 women with peripartum cardiomyopathy, we sequenced 43 genes with variants that have been associated with dilated cardiomyopathy. We compared the prevalence of different variant types (nonsense, frameshift, and splicing) in these women with the prevalence of such variants in persons with dilated cardiomyopathy and with population controls. Results We identified 26 distinct, rare truncating variants in eight genes among women with peripartum cardiomyopathy. The prevalence of truncating variants (26 in 172 [15%]) was significantly higher than that in a reference population of 60,706 persons (4.7%, P=1.3×10(-7)) but was similar to that in a cohort of patients with dilated cardiomyopathy (55 of 332 patients [17%], P=0.81). Two thirds of identified truncating variants were in TTN, as seen in 10% of the patients and in 1.4% of the reference population (P=2.7×10(-10)); almost all TTN variants were located in the titin A-band. Seven of the TTN truncating variants were previously reported in patients with idiopathic dilated cardiomyopathy. In a clinically well-characterized cohort of 83 women with peripartum cardiomyopathy, the presence of TTN truncating variants was significantly correlated with a lower ejection fraction at 1-year follow-up (P=0.005). Conclusions The distribution of truncating variants in a large series of women with peripartum cardiomyopathy was remarkably similar to that found in patients with idiopathic dilated cardiomyopathy. TTN truncating variants were the most prevalent genetic predisposition in each disorder.
Source: N Engl J Med. 2016 Jan 21;374(3):233-41. doi: 10.1056/NEJMoa1505517. Epub 2016 Jan 6.
GRANT OF THE MONTH
PI: Xinyan Tracy Cui
Title: Inhibition of Neural Electrode-mediated Inflammation and Neuronal Cell Death
Description: Inhibition of Neural Electrode-mediated Inflammation and Neuronal Cell Death A growing number of implantable neural electrode devices are being developed to map brain circuit or restore function and treat diseases. The performance of these devices hinges on the quality and stability of the electrode-neural tissue interface. Undesirable brain tissue responses, including persistent microglia activation and blood brain barrier breach, glial scarring, neuronal loss and degeneration, have been consistently reported in animal studies. For electrode devices that require intimate contact with host neurons, their performance functionality may be compromised by these responses. As an example, single unit neural recording via microelectrode arrays experiences deterioration in yield and quality over time, which is a major barrier to applications of this technology in long-term neuroscience research and clinical translation. There are many molecules and pathways involved in inflammation and neuronal death. We began our study by focusing on caspase-1, as caspase-1 is a key mediator of both inflammation and programmed cell death. Activation of caspase-1 is the earliest detectable event in neuronal apoptosis in vitro and in brains with ischemic, injury and neurodegenerative conditions. Furthermore, caspase-1 activates interleukin-1 (IL-1), a pro-inflammatory cytokine highly expressed in the tissue surrounding implanted electrodes, especially those that showed poor electrophysiological outcome. IL-1 triggers inflammatory gliosis and exacerbates BBB breach;both are hypothesized causes of chronic recording failure. Therefore, we hypothesize that caspase-1 mediates the neuronal death and inflammation around neural implants and inhibiting caspase-1 may improve neuronal survival, reduce inflammation and lead to improved electrode performance. We have performed a preliminary study comparing the neural recording performance of microelectrode arrays implanted in caspase- 1 knockout (KO) vs. wild-type (WT) mice. The single unit yield and signal quality are significantly greater in the knockout animals over the 6 month time period, strongly supporting the critical role for caspase-1 in maintaining the quality of the electrode-tissue interface. However, closer examination of the recording over time revealed dynamic changes that cannot be interpreted with end-point histology. To better understand the mechanism(s) by which caspase-1-mediated pathways affect recording, we propose to use 2-photon live animal imaging to characterize the cellular and vascular responses to implanted neural probes in conjunction with neural recording and comprehensive tissue and biochemical analyses. Therapeutics targeting caspase-1 or the inflammation/cell death in general will be evaluated in an effort to improve the chronic neural interface. The drugs to be tested are caspase 1 specific inhibitor VX765, melatonin and minocycline. This proposal uses a multidisciplinary approach to uncover the molecular and cellular mechanism contributing to neural recording performance. The findings will increase our scientific understanding of neural implant pathology, and guide the development of therapeutic and/or biomaterial strategy for stable and reliable neural interface. Data and technology developed in this project may also contribute to the study of neuronal degeneration and inflammation in traumatic brain injury, stroke and neural degenerative diseases.
Public Health Relevance: Neural probes are microelectrode devices implanted in the brain for understanding the brain circuitry or treating neurological disorders. This project will investigate the fundamental role of caspase-1 pathways in mediating host tissue responses to implanted neural electrode devices. Therapeutics will be administered to inhibit the neuronal death and inflammation for improved electrode performance.
Source: National Institute of Neurological Disorders and Stroke (NINDS)
Term: 07/01/2015 – 06/30/2020
Amount: $3.1 million

