February 2019 | VOL. 18, NO. 02| www.McGowan.pitt.edu
Celebrating a Milestone—Dr. Stephen Badylak’s H-index Reaches 100

Congratulations to McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery at the University of Pittsburgh and director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute, as his h-index (Scopus) has reached 100. The h-index is an author-level metric that attempts to measure both the productivity and citation impact of the publications of a scientist. The index is based on the set of the scientist’s most cited papers and the number of citations that they have received in other publications.
This is a prestigious accomplishment. A recent study identified that there were only 3,160 researchers world-wide (living and deceased) that had an h-index (Google Scholar) greater than 100. [Note: There are different services that calculate the h-index. On Scopus, Dr. Badylak’s h-index is 100; on Google Scholar, his h-index is 119.] The study identified 21 other researchers across the University of Pittsburgh with a h-index that exceeded 100. Other McGowan Institute for Regenerative Medicine affiliated faculty members who have also achieved this same success include: Krzysztof Matyjaszewski, PhD; Timothy Billiar, MD; Simon Watkins, PhD; Anthony Demetris, MD; John Kellum, MD; Rocky Tuan, PhD; and Derek Angus, MD.
There are 418 cited publications that Dr. Badylak has authored/co-authored, and those publications have been cited over 46,000 times. Some of the most cited publications in Dr. Badylak’s bibliography are:
“A perivascular origin for mesenchymal stem cells in multiple human organs”
Cell Stem Cell, 2008. 3 (3): 301-313. PMID: 18786417
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak SF, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. (2,175 citations)
“Decellularization of tissues and organs”
Biomaterials; Volume 27, Issue 19, July 2006, Pages 3675-3683 PMID: 16519932
Gilbert, T.W., Sellaro, T.L., Badylak, S.F (1,123 citations)
“An overview of tissue and whole organ decellularization processes”
Biomaterials; Volume 32, Issue 12, April 2011, Pages 3233-3243 PMID: 21296410
Crapo, P.M., Gilbert, T.W., Badylak, S.F. (1,063 citations)
“Extracellular matrix as a biological scaffold material: Structure and function”
Acta Biomaterialia; Volume 5, Issue 1, January 2009, Pages 1-13 PMID: 26235342
Badylak, S.F., Freytes, D.O., Gilbert, T.W. (899 citations)
“Macrophage phenotype as a determinant of biologic scaffold remodeling”
Tissue Engineering Part A; 2008. 14 (11): 1835-1842. PMID: 18950271
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (407 citations)
Congratulations are extended to Dr. Badylak on this well-deserved recognition of his pioneering achievements in tissue engineering and regenerative medicine!
RESOURCES AT THE MCGOWAN INSTITUTE
March Histology Special
The expression of the human Ki-67 protein is strictly associated with cell proliferation. During interphase, the antigen can be exclusively detected within the nucleus, whereas in mitosis most of the protein is relocated to the surface of the chromosomes. The fact that the Ki-67 protein is present during all active phases of the cell cycle (G(1), S, G(2), and mitosis), but is absent from resting cells (G(0)), makes it an excellent marker for determining the so-called growth fraction of a given cell population.
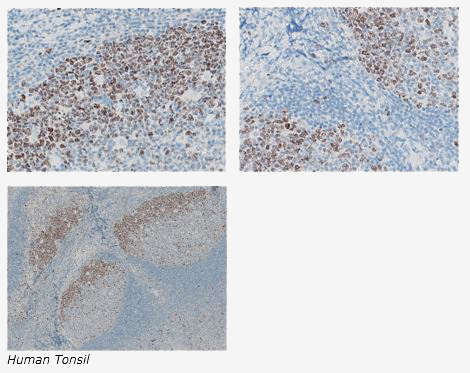
The Histology Lab at The McGowan Institute offers competitive pricing for IHC staining, including “working up” new protocols for Paraffin and Frozen sections. We have Ki67 already up and running and will waive the work up fee. Bring your slides, blocks, frozen or fixed tissue to the histology lab for Ki67 staining. You are guaranteed beautiful results.
Contact the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology work with the quickest turn-around time.
UPCOMING EVENTS
McGowan Retreat and Open Session
Faculty, Trainees and, Staff: This your last opportunity to register for the March 11 & 12, 2019 scientific retreat. Click Here for program details.
Also, many may be interested in attending the open-to-the-public panel discussion “The Hope vs. Hype of Regenerative Medicine” followed by “Meet the Researchers of the McGowan Institute” reception and poster session on March 12, 2019. Click Here for program details.
SCIENTIFIC ADVANCES
Pitt-led Research Describes How Neurons Could Disconnect From Each Other in Huntington’s Disease

A hallmark of neurodegenerative diseases like Huntington’s is the progressive death of nerve cells in the brain. The cells don’t die quickly, though. They first start to disconnect from each other because their neurites — long finger-like extensions that make connections all through the brain — become smaller.
Now, using animal models and nerve cells grown in the lab, researchers from the University of Pittsburgh School of Medicine suggest a new mechanism dubbed “neuritosis” that might explain neurons shrinking in Huntington’s and other neurodegenerative diseases, opening new targets for therapy. The study is published this week in the journal Proceedings of the National Academy of Sciences and includes these co-authors affiliated with the McGowan Institute for Regenerative Medicine:
- Takashi Kozai, PhD, Assistant Professor in the Department of Bioengineering at the University of Pittsburgh
- Tracy Cui, PhD, William Kepler Whiteford Professor of Bioengineering at the University of Pittsburgh and the Director of the Neural Tissue/Electrode Interface and Neural Tissue Engineering Lab
- Valerian Kagan, PhD, DSc, Professor and Vice-Chairman in the Department of Environmental and Occupational Health as well as a Professor in the Department of Pharmacology and Chemical Biology, the Department of Radiation Oncology, and the Department of Chemistry at the University of Pittsburgh
“Neuritosis is a process that hasn’t been recognized or described until now and could play a very important role in normal brain development, aging and neurodegenerative disease,” said senior author Robert Friedlander, MD, chair and Walter E. Dandy Professor of Neurosurgery and Neurobiology at Pitt’s School of Medicine.
It all started when Sergei Baranov, PhD, a staff scientist in Friedlander’s lab, noticed an interesting phenomenon in mouse nerve cells that he was growing in the lab.
“Their mitochondria, the cellular powerhouses, weren’t working as well at the neurite ends,” said Dr. Baranov. Then, when the researchers looked at neurons in the spinal cords of mice, they found the same phenomenon. “Other researchers must have noticed this as well, but we were able to visualize it and suggest the potential cause as well as outcome,” he noted.
The researchers found that when proteins in mitochondria at the ends of neurites were damaged by normal wear and tear, newer ones weren’t coming in to replace them as quickly as they did for mitochondria near the nucleus. This made them function less efficiently, which activated ‘executioner’ enzymes called caspases and ultimately led to neurites withering away.
Dr. Friedlander and his team called this neuritosis, a variation on apoptosis, the cell death process that involves caspase activation. “It’s quite intuitive, once we discovered how it works,” said Dr. Friedlander. “Neuronal projections are really long and the farther you are from the nucleus, which is the central factory, the harder it gets to repair and replenish the cellular machinery, making the ends more vulnerable to even small stresses.”
The researchers then wanted to understand whether neuritosis plays a role in neurodegenerative disease, and they had reason to suspect that it does. Previous work in Dr. Friedlander’s lab showed that mutations in huntingtin, the protein linked to Huntington’s, interferes with the same protein supply chain that breaks down in neuritosis.
To test their hunch, Dr. Friedlander’s team used genetically modified mice that carried a mutant version of the human huntingtin protein. These mice exhibit symptoms of the disease, including accelerated neuronal death. Their findings were similar to what they had seen in the cells, but more pronounced. There were fewer mitochondria at the ends, and what remained was more dysfunctional than in normal neurons. There was also more activation of caspases, and increased levels of cell death.
“It’s quite likely that neuritosis happens in nerve cells normally and doesn’t result in cell death, but in neurodegenerative diseases, there are higher levels of stress to the already vulnerable neurite ends, which could push neuritosis over the edge and lead to cells dying,” said Dr. Friedlander. “If we can somehow find a way to keep mitochondria at nerve ends healthy, it may be beneficial in treating neurodegenerative diseases.”
Additional authors on the study include Oxana V. Baranova, Svitlana V. Yablonska, PhD, Yalikun Suofu, PhD, Alberto L. Vazquez, PhD, Lisa M. Ferrando, Timothy M. Larkin, Yulia Y. Tyurina, PhD, Diane L. Carlisle, PhD, all of Pitt, and Bruce S. Kristal, PhD, of Harvard Medical School.
The research was funded by National Institute of Health grants R01NS089688, R01NS039324, R01NS077748 and R01NS100743, the David Scaife Family Charitable Foundation, the University of Pittsburgh Brain Institute, and the Walter L. Copeland Fund of the Pittsburgh Foundation.
Anti-Rejection Drug Could Be Repurposed to Treat Cancer
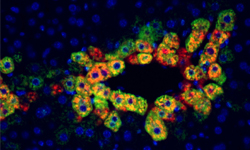
In animal models and patient tissues, researchers from the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty members Satdarshan “Paul” Monga, MD, professor of pathology and the founding director of the Pittsburgh Liver Research Center at Pitt’s School of Medicine, and Michael Oertel, PhD, assistant professor of pathology and a member of the Pittsburgh Liver Research Center —have identified a new molecular pathway in the liver that suggests a commonly used anti-rejection medication could be repurposed to treat certain liver cancers.
“What we’ve found is that liver cancers with a specific mutation in the β-catenin gene are possibly more susceptible to rapamycin, a commonly used anti-rejection medication in transplantation,” said Dr. Monga, senior author of the study published in Cell Metabolism. “We think this gives us a new precision medicine approach to develop therapies for liver cancer, which often are very resistant to treatment.”
The current study began when Dr. Monga and his team made a serendipitous observation that a ring of cells surrounding the central vein, a blood vessel found within the liver, showed high levels of a protein called mTOR. The active mTOR protein, a nutrition and energy sensor central to cellular metabolism, was found in the same cells where β-catenin was known to be active.
Approximately 20 to 35 percent of liver cancers have a β-catenin mutation, but there is little understanding of how and why these mutations could be aiding the growth of cancerous cells. So, when Monga found similarly high levels of active mTOR in β-catenin mutated liver cancers, he wondered whether the two proteins could be functionally linked.
To trace the source of mTOR activation, the scientists created a mouse model of liver cancer in which they genetically mutated β-catenin, along with another gene called Met, specifically in liver cells. These mice developed liver cancers that were genetically very similar to those seen in humans.
Starting with increased mTOR activation, they followed the molecular breadcrumbs in these mice to show that β-catenin activated mTOR through an intermediate enzyme called glutamine synthase (GS). This molecular pathway was linked to energy intake, as fast-growing cancer cells, which consume more energy than normal cells, showed higher activity of GS and mTOR.
“I like to say these tumors are mTOR addicted,” said Dr. Monga, who also is an investigator at the UPMC Hillman Cancer Center. “Activating mTOR kicks up the protein-making factories in these cells, giving them the resources to divide and grow.”
When the genetically modified mice were fed rapamycin – an immunosuppressant that inhibits mTOR – the tumors decreased in size, and when they added another drug that inhibited Met, the tumors were almost completely killed, showing that mTOR played an important role in helping these tumors grow.
The researchers note that a previous clinical trial in patients with liver cancer did not find a significant benefit for rapamycin, but the new findings suggest that perhaps if the pool were limited to patients with β-catenin mutations and mTOR-addicted tumors, the treatment would have been more successful.
“Current liver cancer therapies increase the likelihood of survival only by 3 or 4 months, so taking a precision medicine approach to identify the right patient could allow us to repurpose existing drugs to improve treatment success,” Dr. Monga said.
Beyond treating liver cancer, the study also points to ways in which we could reduce the risk of cancer recurrence in some patients undergoing liver transplant.
“While transplant patients are prescribed either rapamycin or a different immunosuppressant, those that have β-catenin mutated and mTOR-addicted tumors might benefit from using rapamycin as the preferred anti-rejection medication. We hope to conduct clinical trials in the near future to test rapamycin both in treating liver cancer and to prevent its recurrence in patients receiving liver transplants,” Dr. Monga said.
Illustration: Mouse liver tissues showing cells surrounding the central vein with active mTOR (red) and glutamine synthetase (green) being present in the same cells (yellow). Credit: Cell Metabolism/University of Pittsburgh.
Addendum: Rapamycin (sirolimus) is also being evaluated by McGowan affiliated faculty member Marc A. Simon, MD, MS, FACC, Associate Professor of Medicine, Bioengineering, and Clinical Translational Science to treat severe pulmonary arterial hypertension. Dr. Simon, his colleagues, and Aadi Bioscience, Inc, have designed and are running a phase I dose finding multicenter study of nanoparticle albumin-complexed sirolimus. Additional information on the clinical trial is available at Clinicaltrials.gov NCT02587325. Initial results will be presented at several upcoming meetings: American College of Cardiology, the International Society for Heart and Lung Transplantation and the American Thoracic Society. This novel formulation of the drug is also under study for cancer therapy.
Peripartum Cardiomyopathy Awareness

The Peripartum Cardiomyopathy Network (PCN) is a network of physicians and nurses at clinical sites across the United States and Canada dedicated to both clinical care and investigation of peripartum cardiomyopathy. McGowan Institute for Regenerative Medicine affiliated faculty member Dennis McNamara, MD, Professor of Medicine, University of Pittsburgh and the Director of the Heart Failure/Transplantation Program at the University of Pittsburgh Medical Center, is a co-director of PCN.
Peripartum cardiomyopathy (PPCM) is a rare complication of pregnancy which occurs in 1 of every 3,000-4,000 live births in the United States. It shares all the phenotypic features of other forms of acute non-ischemic cardiomyopathy but presents in women in the last month of pregnancy or the first several months postpartum. While most affected women will recover varying levels of cardiac function over the first year after presentation, unfortunately almost half do not recover completely and are left with chronic dilated cardiomyopathy. Peripartum cardiomyopathy remains a major cause of maternal morbidity and mortality.
Recently, Amanda Roberts for Fox8 highlighted PPCM and obtained input from Dr. McNamara for her report.
Dr. McNamara says the biggest problem surrounding PPCM is the lack of awareness of the condition among medical providers.
“No one expects a young woman in her 20s, 30s, 40s to be presenting with a heart condition at a time that should be a wonderful event – the birth of their child. It’s unexpected, and symptoms can be quite subtle,” Dr. McNamara said.
While the condition can be genetic, doctors say it can also develop in any pregnant woman and tends to show up more in black women.
“It’s sad to say, but it’s an important issue right now because the U.S. is the only developed country where maternal mortality is actually increasing, and there’s a big effort now to find out why that is and how that can be changed,” Dr. McNamara said.
The tricky thing is that the symptoms of PPCM mimic those experienced during pregnancy and after delivery. Dr. McNamara says that’s why women themselves are their best advocate. If a woman experiences excessive swelling, shortness of breath, difficulty breathing or heart palpitations, she should go to the doctor asking specifically about her heart.
Dr. McNamara says the test to confirm PPCM is relatively easy with a non-invasive echocardiogram. And doctors say once it’s diagnosed early, it’s easier to treat. Dr. McNamara says they continue to make medical providers more aware.
“We are getting better. People are picking up and are more aware of the illness. It’s relatively easy to diagnose if you think of it. The important thing is to think of it,” he said.
Dr. McNamara says there are women whose cases of PPCM are not caught early enough, which results in the mother needing a transplant or death. But for those cases that are caught early, roughly 70 percent of those women have their heart function return to normal over the course of a year.
The Matrix
The Badylak Lab was featured recently on the weekly TV news show, “Full Measure”. McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery at the University of Pittsburgh and Director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute, was interviewed by Joce Sterman, an investigative journalist whose work has won her numerous awards including regional Emmys, Associated Press recognition, and an Edward R. Murrow award for investigative contributions to breaking news.
“The Matrix” focuses on how modern wars are creating a new class of wounded warrior. IEDs can leave troops with horrific injuries…that they survive. Now there are new ways to heal the broken bodies that is almost like science fiction. Ms. Sterman talks with both Marine Sergeant Ronald Strang and Dr. Badylak and they show viewers a treatment that’s aptly called the matrix. Viewers learned how the Badylak Lab is using a regenerative medicine approach to reconstitute functional musculotendinous tissue.
Full Measure is a weekly Sunday news program focusing on investigative, original and accountability reporting. The host is Sharyl Attkisson, five-time Emmy Award winner and recipient of the Edward R. Murrow award for investigative reporting. She is backed by a team of award-winning journalists.
Scientists ‘See’ Dual-Layered Scaffolding of Cellular Nuclei
Our cells sometimes have to squeeze through pretty tight spaces. And when they do, the nuclei inside must go along for the ride. Using super-sensitive microscopic imaging, a team of scientists from the University of Pittsburgh and Carnegie Mellon University have made a fundamental biological discovery that explains the structure of the nuclear envelope and gives tantalizing clues as to how cells squish through narrow openings without springing a leak.
The findings, which also could be key to untangling the mechanisms underlying several genetic diseases, are described in the Proceedings of the National Academy of Sciences. McGowan Institute for Regenerative Medicine faculty member Donna Stolz, PhD, Associate Director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an Associate Professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is a co-author of the study.
“It’s quite the serendipitous discovery,” said Quasar Padiath, MBBS, PhD, associate professor in the Pitt Graduate School of Public Health’s Department of Human Genetics and one of the senior authors on the research. “Just like everyone else, I thought we knew how the cellular nuclear envelope was organized, but as we took a closer look while investigating a genetic condition, we found that there was far more to the story.”
Every animal cell contains a nucleus, home to the majority of its genetic material. Lining the interior of the membrane encasing the nucleus is the nuclear lamina, a scaffold that gives the nucleus its spherical structure. Scientists had previously shown the lamina to be formed by a tangled meshwork of filaments, made up of proteins called lamin A and B.
Dr. Padiath teamed up with Yang Liu, PhD, associate professor in Pitt’s departments of medicine and bioengineering, to take a closer look at the nuclear lamina because people with a fatal genetic condition he studies – autosomal dominant leukodystrophy with autonomic disease (ADLD) – have extra copies of the gene that codes for lamin B1, a subtype of lamin B. The scientists first looked at the lamina in normal cells using a super-resolution imaging technique called “stochastic optical reconstruction microscopy” (STORM).
To their surprise, the team discovered that there are actually two distinct meshworks – an outer, more loosely woven layer of lamin B and an inner, tighter layer of lamin A.
“It is truly remarkable that STORM is able to visualize such a microscopically small separation between lamin A and B1,” said Dr. Liu, who also is a researcher at the UPMC Hillman Cancer Center. “That has never been seen with conventional light microscopy.”
Dr. Padiath’s team then built on an ongoing partnership with Kris Dahl, PhD, a Carnegie Mellon University professor of chemical engineering who studies the mechanics and architecture of nuclear membranes, to learn about how the lamin layers function. By imaging nuclei under varying degrees of pressure, the scientists discovered that when a cell is compressed, the outer, more loosely woven lamin B1 layer thins, allowing the lamin A layer to bulge out at the axes of the nucleus.
“For me, this process is similar to one of my knitting projects,” said Dr. Dahl. “Based on the holes between the stitches and the thickness of the yarn, you can predict the stiffness of the material.”
The scientists believe their observations indicate that the distinct lamin layers are part of a necessary cellular system: When functioning correctly, it allows nuclei to relieve pressure when compressed by biologic functions – such as moving within a very thin blood vessel or squeezing through a narrow opening – to avoid damage to the nucleus itself.
In the disease that Dr. Padiath studies – ADLD – patients typically live into their 40s and 50s before experiencing symptoms tied to fatal brain degradation. Because ADLD involves extra copies of the lamin B1 gene, Dr. Padiath’s future work will explore how excessive lamin B could negatively impact brain cells at middle age.
“Now that we can look at the nuclear architecture in such exquisite detail, we can start asking, ‘How does it change in ADLD and other lamina diseases, particularly with aging?’” Dr. Padiath said.
Additional authors on this paper are Bruce Nmezi, BS, Jianquan Xu, PhD, Rao Fu, PhD, Guillermo Bey, PhD, Juliana Powell, BS, Hongqiang Ma, PhD, and Mara Sullivan, BS, all of Pitt; Travis Armiger, PhD, of Carnegie Mellon University; Yiping Tu, Stephen Young, MD, and Natalie Chen, BS, all of the University of California, Los Angeles.
This work was supported by National Institutes of Health grants R01NS095884, EB003392, R01EB016657, R01CA185363, 1S10RR019003-01 and 1S10RR025488-01; National Multiple Sclerosis Society grant 5045A1, and National Science Foundation grant CMMI-1634888.
Illustration: Frame from animation of discovery. UPMC.
Clinical Trial: Withdrawal of Immunosuppression in Living Donor Liver Transplantation

McGowan Institute for Regenerative Medicine affiliated faculty member Abhinav Humar, MD, Clinical Director of the Thomas E. Starzl Transplantation Institute, Chief, Division of Transplantation in the Department of Surgery at the University of Pittsburgh Medical Center, Professor in the Department of Surgery at the University of Pittsburgh School of Medicine, and a Staff Physician at the Pittsburgh VA Medical Healthcare System, is the principal investigator of the clinical trial entitled, “Safety and Preliminary Efficacy of Donor-derived Regulatory Dendritic Cell (DCreg) Infusion and Immunosuppression Withdrawal in Living Donor Liver Transplantation” (ClinicalTrials.gov Identifier: NCT03164265).
Gina Kolata is a New York Times writer who tells the story of Dr. Humar’s first clinical trial participant and the patient’s experience over the past two years. She notes, “Patients receiving new kidneys and livers must take damaging anti-rejection drugs for the rest of their lives. Now researchers hope to train the immune system instead of just tamping it down.”
“You are trying to fool the body’s immune system,” Dr. Humar said. “That is not easy to do.”
The Phase I/II cohort study includes low risk living donor liver transplant recipients who will receive a single infusion of donor-derived DCreg with concurrent mycophenolic acid (MPA) therapy (1/2 dose) one week prior to transplantation. All patients will be maintained on MPA and Tacrolimus (TAC, an immunosuppression drug) for the first six months after transplantation. At that time point, recipients meeting specific criteria (no rejection and permissive liver function tests (LFTs)) will be slowly weaned off MPA per standard of care over a period of six months. Participants will then be evaluated for TAC weaning at one year after transplantation. Those who meet the criteria of no rejection and permissive LFTs will undergo a protocol liver biopsy and proceed to TAC weaning over six months if liver biopsy is permissive. Successfully weaned participants who remain rejection-free will undergo 3 years of follow-up after the last dose of immunosuppression. They will undergo a liver biopsy at one and three years after immunosuppression withdrawal. Participants who are removed from the study protocol at any time will return to standard of care but will continue to be followed by the study team and will undergo a liver biopsy at the end of the study (4.5 years after transplantation).
Angus Thomson, PhD, DSc, Distinguished Professor of Surgery and Immunology, University of Pittsburgh, is the study’s responsible party.
Three Novel Biomedical Projects Receive Center for Medical Innovation Awards Round-2 2018 Pilot Funding

The University of Pittsburgh’s Center for Medical Innovation (CMI) awarded grants totaling $60,000 to three research groups through its 2018 Round-2 Pilot Funding Program for Early Stage Medical Technology Research and Development. The latest funding proposals include a new drug-eluting contact lens for treatment of dry eye disease, a new method of measuring ocular changes in glaucoma, and a new instrument for management of ketogenic diets. Each of these projects includes McGowan Institute for Regenerative Medicine affiliated faculty members on their research teams.
This is our eighth year of pilot funding, and our leadership team could not be more excited with the breadth and depth of this round’s awardees,” said McGowan Institute affiliated faculty member Alan Hirschman, PhD, CMI Executive Director. “This early-stage interdisciplinary research helps to develop highly specific biomedical technologies through a proven strategy of linking UPMC’s clinicians and surgeons with the Swanson School’s engineering faculty.”
AWARD 1: “Polyelectrolyte Multilayer Coating for Delivery of IL-4 from Contact Lenses for Dry Eye Disease”
For the development of a drug-eluting contact lens for treatment of chronic “dry eye” disease.
- Bryan Brown, PhD, Assistant Professor, Departments of Bioengineering, Obstetrics, Gynecology, and Reproductive Sciences; McGowan Institute for Regenerative Medicine
- Vishal Jhanji, MD, FRCSG, FRCOphth, Professor of Ophthalmology, Cornea, External Eye Diseases and Refractive Surgery Services, UPMC Eye Center
- Mangesh Kulkarni, MD, PhD, Research Assistant Professor, McGowan Institute for Regenerative Medicine and Pitt’s Department of Bioengineering
AWARD 2: “On the quantitative analysis of a new tonometer to manage/prevent glaucoma”
For the development of a novel pulse wave device for measurement of ocular tissue characteristics in the detection and treatment of glaucoma.
- Piervincenzo Rizzo, PhD, Professor, Department of Civil and Environmental Engineering, University of Pittsburgh
- Ian A. Sigal, PhD, Assistant Professor, Department of Ophthalmology, University of Pittsburgh Medical Center, Eye & Ear Institute
- Ian Conner, PhD, MD, Assistant Professor of Ophthalmology, Department of Ophthalmology, University of Pittsburgh
AWARD 3: “Acetone Breathalyzer for Monitoring the Ketogenic State”
For the development of a cost-effective, rapid acetone “breath-alayzer” for clinical and consumer usage in ketogenic diets.
- Sung Kwon Cho, PhD, Department of Mechanical Engineering & Materials Science, Swanson School of Engineering
- David Rometo, MD, Division of Endocrinology and Metabolism, University of Pittsburgh Medical Center
- David Finegold, MD, Department of Human Genetics, Graduate School of Public Health
- Alex Star, PhD, Department of Chemistry, Dietrich School of Arts and Science
CMI, a University Center housed in Pitt’s Swanson School of Engineering (SSOE), supports applied technology projects in the early stages of development with “kickstart” funding toward the goal of transitioning the research to clinical adoption. Proposals are evaluated on the basis of scientific merit, technical and clinical relevance, potential health care impact and significance, experience of the investigators, and potential in obtaining further financial investment to translate the particular solution to healthcare.
Congratulations, all!
Illustration: University of Pittsburgh’s Center for Medical Innovation.
AWARDS AND RECOGNITION
Dr. Adam Feinberg Elected to Leadership Advisory Committee of ARMI

McGowan Institute for Regenerative Medicine affiliated faculty member Adam Feinberg, PhD, Associate Professor in the Departments of Biomedical Engineering and Materials Science and Engineering at Carnegie Mellon University, has been elected to the Leadership Advisory Committee (LAC) for the Advanced Regenerative Manufacturing Institute (ARMI).
The LAC advises BioFabUSA related to policies and strategic guidance concerning technology and research priorities, objectives, and content of research programs, policies and strategic guidance concerning the education and workforce development programs and the strategic direction of BioFabUSA.
Congratulations, Dr. Feinberg!
Illustration: Carnegie Mellon University.
Dr. Ron Poropatich: MTEC New Board Member and Healthcare Transformation Editor

The Medical Technology Enterprise Consortium (MTEC) has named McGowan Institute for Regenerative Medicine affiliated faculty member Ron Poropatich, MD, one of its newest board members. MTEC is a 501(c)(3) biomedical technology consortium collaborating under an Other Transaction Agreement (OTA) with the U.S. Army Medical Research and Materiel Command (USAMRMC) that serves those who serve our nation. In partnership with the Department of Defense (DoD) and private support, MTEC is working to prevent injuries and accelerate the development of revolutionary medical solutions that will enhance wound healing and return the wounded to fully functioning lives. Ultimately, all citizens will benefit from these technologies and health care solutions.
Dr. Poropatich currently serves as the Director of the Center for Military Medicine Research (CMMR) and as a Professor of Medicine in the Pulmonary, Allergy and Critical Care Division at the University of Pittsburgh. Under Dr. Poropatich’s leadership, the CMMR has developed a large DoD medical research portfolio and established the University of Pittsburgh as a key collaborative research partner for the DoD. Since 2012, Dr. Poropatich has also acted as the Senior Advisor on Telemedicine for the University of Pittsburgh Medical Center, leveraging the use of virtual health solutions for this innovative leader in health care delivery.
Dr. Poropatich also was named to the inaugural editorial team of the new Mary Ann Liebert, Inc./Genetic Engineering News journal, Healthcare Transformation. Publishing quarterly online and in print, Healthcare Transformation will provide an exclusive view into effective practices and strategies presented by leaders in the field. Readers can become participants in a unique multimedia platform that allows for dialogue among a community of top academic scientists, corporate innovators, business leaders, and influencers who provide unique and fascinating views on the meaningful actions in transforming our healthcare system for a better future.
Congratulations, Dr. Poropatich!
Dr. Bernard Costello Begins Tenure as Medical Association President

McGowan Institute for Regenerative Medicine faculty member Bernard J. Costello, DMD, MD, dean of the University of Pittsburgh School of Dental Medicine, has begun his year as president of the American Cleft Palate-Craniofacial Association (ACPA).
Dr. Costello, who is also a professor of oral and maxillofacial surgery, said in a recent welcome message that the association will “aim to improve upon the notable successes of the past and innovate for the future.”
“The chance to help lead this organization is a rare privilege and I am humbled to have had the opportunity to work with such fantastic leaders,” he said. “ACPA is filled with people who know teamwork like no other organization that I am a part of.”
Congratulations, Dr. Costello!
Illustration: University of Pittsburgh School of Dental Medicine.
Dr. Thomas Gilbert: Swanson School of Engineering 2019 Distinguished Alumni Award Recipient

Recognizing their impact in industry and government, the University of Pittsburgh’s Swanson School of Engineering announced its 2019 Distinguished Alumni honorees. The recipients, who represent the School’s six departments and the Swanson School overall, will be honored at the 55th annual Distinguished Alumni Banquet on March 28, 2019, at 5:30 pm in the Connolly Ballroom of Alumni Hall.
This year, McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gilbert, PhD, Chief Science Officer, Research & Development, Clinical Research, at ACell, Inc., Columbia, MD, and an Adjunct Assistant Professor, Department of Bioengineering, at Johns Hopkins University, Baltimore, MD, was named a Distinguished Alumni Award recipient. Dr. Gilbert was a Bioengineering Department graduate in 2006.
The Distinguished Alumni Award is presented annually to Swanson School alumni who have demonstrated outstanding professional achievement in their respective fields of engineering.
“For more than 170 years, Pitt’s engineering graduates have contributed greatly to engineering disciplines, as well as to the betterment of everyday life,” noted James R. Martin II, U.S. Steel Dean of Engineering.
Congratulations, Dr. Gilbert!

CMU’s Drs. Cohen-Karni and Whitehead Awarded Dean’s Early Career Fellowships
Congratulations to McGowan Institute for Regenerative Medicine affiliated faculty members Tzahi Cohen-Karni, PhD, Assistant Professor in the Department of Biomedical Engineering at Carnegie Mellon University (CMU), and Kathryn Whitehead, PhD, Assistant Professor in the Departments of Chemical Engineering and Biomedical Engineering at CMU, who have been awarded the 2019 Dean’s Early Career Fellowships for cutting-edge innovations in their fields and outstanding contributions to the college and university. Dean’s Early Career Fellowships are awarded to untenured faculty members who have been nominated by their department heads and then selected to receive the fellowship after review and discussion of the nomination package by the CIT Review Committee.
Congratulations, Drs. Cohen-Karni and Whitehead!
Illustration: Carnegie Mellon University.
Campbell Lab Student Receives Award and Scholarship

Carnegie Mellon University (CMU) Biomedical Engineering (BME) PhD student Saigopalakrishna “Sai” Yerneni, co-advised by McGowan Institute for Regenerative Medicine affiliated faculty members Phil Campbell, PhD, Research Professor at the Institute for Complex Engineered Systems at the Carnegie Institute of Technology (School of Engineering) at CMU, and Lee Weiss, PhD, Emeritus Research Professor, CMU, is the recipient of a competitive 2019 Young Investigator Award and Scholarship from the International Society for Extracellular Vesicles (ISEV) for his work on extracellular vesicles in host-pathogen interactions. As a part of the award, he will be giving a talk at the ISEV 2019 annual meeting in Kyoto, Japan.
Congratulations, Mr. Yerneni!
Illustration: Carnegie Mellon University.
Important Upcoming Dates
ASAIO 65th Annual Conference
Abstract Submission Deadline is February 7th, 2019
Click Here to submit
ASAIO Student Design Competition
Deadline for Preliminary Proposals is February 11, 2019
Click Here for competition guidelines
Regenerative Medicine Summer School
Registration is now open!
Click Here for more information
Pittsburgh Life Sciences Week: May 13-17, 2019
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#193 –– Drs. Julie Phillipi and Bryan Brown discuss the upcoming 2019 McGowan Institute Scientific Retreat and the Open-to-the-Public Forum, “Hope vs. Hype of Stem Cell Therapy.”
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK
Title: The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships
Summary: BACKGROUND: The Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs), a joint effort among the National Heart, Lung, and Blood Institute, the Food and Drug Administration, the Centers for Medicare and Medicaid Services, and others, was established in 2005 at the University of Alabama at Birmingham. The registry examined clinical outcomes and quality-of-life metrics of patients who received an Food and Drug Administration-approved durable mechanical circulatory support (MCS) device to treat advanced heart failure. On January 1, 2018, the Intermacs Database became part of The Society of Thoracic Surgeons National Database, providing additional resources for quality assessment and improvement and scientific advancement.
METHODS: The Intermacs Database Annual Report summarizes outcomes in patients (≥19 years of age) who underwent durable MCS implant between June 23, 2006, and December 31, 2017. Outcomes are presented for patients who underwent isolated continuous flow left ventricular assist device (CF LVAD) support, CF LVAD support with concomitant right ventricular assist device (RVAD) implant, or total artificial heart implant. Analyses of patients with CF LVADs are stratified by axial flow and centrifugal flow configurations. Because of the association of era with outcomes, the survival analyses are restricted to isolated CF LVADs implanted in the 2012 to 2016 era.
RESULTS: There were 25,145 adult patients with MCS reported to Intermacs, of whom 18,539 (74%) received CF LVADs, 667 (2.6%) had an RVAD with CF LVAD, 339 received a total artificial heart (1.3%), and 20 (0.07%) received an isolated RVAD. Of the CF LVADs, mean age was 57 ± 1 years, 26% were listed for transplantation, and 51% were in cardiogenic shock (profile 1 to 2) preoperatively. CF LVADs included 14,527 axial flow (78%) and 4,012 centrifugal flow (22%) devices. Intermacs patient phenotype has evolved over time to include more patients with profile 3 (26% in 2006 to 2011 versus 35% in 2012 to 2016) and fewer patients with profile 2 (40% versus 35%), patients with better markers of preoperative renal and hepatic function, and more patients who received implants for destination therapy (29% versus 48%) indication. In 2017, centrifugal flow implants (51%) approximated that of axial flow devices (49%). Mean CF LVAD support duration was 20 months (31,563 patient-years). One-year survival for isolated CF LVADs was 83% and 5-year survival was 46%. One-year survivals for centrifugal versus axial flow devices were 85% and 84%, respectively. Patients who required concomitant RVAD support had 1- and 5-year survivals of 58% and 28%, respectively. Freedom from all-cause readmission was 70% at 1 month and 20% at 1 year. At 1 year, stroke occurred in 20% of patients on centrifugal flow and 13% of patients on axial flow support (p < 0.001), gastrointestinal bleeding affected 20% of patients with centrifugal flow devices and 25% of patients with axial flow devices (p < 0.001), and pump-related infection occurred in 28% of patients with centrifugal flow devices versus 25% of patients with axial flow devices (p = 0.01). Neurologic dysfunction (19% of deaths) and multisystem organ dysfunction (15%) were the most common causes of death.
CONCLUSIONS: With the evolution of MCS, patient phenotype and outcomes are also changing over time. CF LVAD support is increasingly being used in the less ill patient phenotype and more patients are supported for destination therapy. Mean survival is now approaching 5 years, but adverse events, especially neurologic events, continue to have a detrimental impact on the success of CF LVAD support.
Source: Journal of Heart and Lung Transplantation. 2019 Feb;38(2):114-126.
GRANT OF THE MONTH
PI: George Gittes
Title: Alpha Cells Conversion to Beta Cells in Non-Human Primates
Description: An ideal solution to the treatment or cure of type 1 diabetes mellitus would be the formation of new functioning β-cells from the patient’s own tissues that are not attacked by the autoimmunity, thereby avoiding the need for any immunosuppression. Abundant recent data have suggested that α-cells are a viable potential source for endogenous transdifferentiation into β-cells. Here, we describe a pancreatic intraductal viral delivery system in the mouse, wherein a single infusion of an adeno-associated virus (AAV), carrying a pdx1/mafA expression vector, is given to a toxin-induced (alloxan) diabetic mouse. This AAV gene therapy induced robust and durable α-cell transdifferentiation into β-cell-like cells through neogenesis, with recovery of over 60% of the β-cell mass within 4 weeks, and with persistent, durable euglycemia. Serendipitously, when this β-cell-like cell neogenesis was similarly induced in the autoimmune NOD mouse model, the mice became euglycemic for 4 months or more, without any additional therapy or immunosuppression. To our knowledge, no clinically applicable β-cell replacement therapy in NOD mice has been successful without immunosuppression. We suspect that the neogenic β-cell-like cells may not be attacked by the autoimmunity because they are “imperfect” β-cells by RNA-seq analysis. Since pancreatic duct injection is routinely performed in humans as a relatively simple, non-surgical procedure, and since numerous viral gene therapy trials are currently ongoing for several diseases, we feel that our approach may be rapidly translatable to humans with type 1 diabetes mellitus. In this proposal, we will perform important proof-of-principle studies in non-human primates as last steps in preparation for human gene therapy clinical trials. The primate pancreas has a very different texture and consistency than the mouse pancreas (and is very similar to the human pancreas). Thus, the mechanics of the viral delivery will likely require substantial alterations. In addition, a glucagon promoter is preferable to the CMV promoter for expression of pdx1 and mafA, so we will strive to develop and optimize a glucagon promoter vector that is effective in primates. Further studies will investigate this pancreatic ductal infusion approach in the context of AAV neutralizing antibodies. We will also perform detailed analyses of the new β-cell-like cells, including physiology, gene expression phenotype, and anatomy. In summary, we feel that the proposed studies, if successful, should position us well in preparation for clinical trials in humans with type 1 diabetes mellitus.
Source: National Institute of Diabetes and Digestive and Kidney Diseases
Term: September 30, 2018 – August 31, 2020
Amount: $391,250
