October 2018 | VOL. 17, NO. 10| www.McGowan.pitt.edu
Technologies from the Badylak Lab Licensed for Development

ECM Therapeutics, Inc. has licensed multiple extracellular matrix (ECM) technologies developed in the laboratory of Stephen Badylak, DVM, PhD, MD (pictured top), including hydrogels, bioactive derivatives and methods for delivering these materials within the body. Dr. Badylak is a professor in the Department of Surgery, a deputy director of the McGowan Institute for Regenerative Medicine, and the director of the Center for Pre-Clinical Tissue Engineering within the Institute.
The Pittsburgh-based company will initially develop EsophaGel™, an ECM hydrogel for the treatment of Barrett’s Esophagus which is often a precursor to esophageal cancer. EsophaGel has been shown in pre-clinical studies to halt and possibly reverse the progression of esophageal cancer.
“We are pleased to license this portfolio of patents and patent applications to a startup company based in the Pittsburgh region,” said Alex Ducruet, PhD, CLP, director for licensing and intellectual property in Pitt’s Innovation Institute. “Dr. Badylak is one of Pitt’s most prolific innovators, and we look forward to the positive impact that these regenerative medicine technologies will have on people’s lives.”
ECM Therapeutics was founded by Dr. Badylak and business development colleague Katie Collins. Badylak lab members Jenna Dziki (pictured bottom) and George Hussey, PhD (pictured center) and the University of Pittsburgh hold equity in the company.
RESOURCES AT THE MCGOWAN INSTITUTE
November Histology Special
Reticular fibers, or reticulin is a type of fiber in connective tissue, composed of type III collagen secreted by reticular cells. Reticular fibers crosslink to form a fine meshwork (reticulin). This network acts as a supporting mesh in soft tissues such as liver, bone marrow, and the tissues and organs of the lymphatic system.
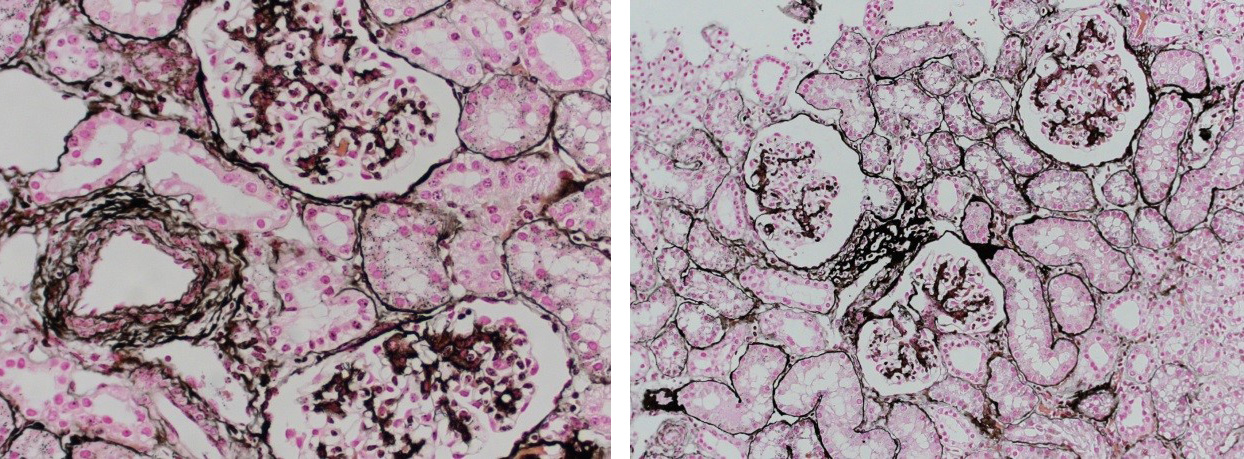
Jones Silver Stain is used to identify basement membrane and reticulin.
You’ll receive 25% off Jones Silver Stain in November when you mention this ad. Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or call 412-624-5265. As always, you will receive the highest quality histology in the lowest amount of time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how.
SCIENTIFIC ADVANCES
Engineering Breast Milk to Treat Sick Infants

McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, assistant professor of chemical engineering at Carnegie Mellon University (CMU), has been awarded a 2018 National Institutes of Health (NIH) Director’s New Innovator Award for her project titled “Fate, Function, and Genetic Engineering of Breast Milk Cells for Infant Therapy.” NIH Director’s Awards are prestigious awards given to exceptionally creative scientists proposing high-risk, high-impact research.
With this award, Dr. Whitehead, who has a courtesy appointment in CMU’s biomedical engineering, will genetically engineer the human cells in breast milk for infant disease therapy—something no one has ever done before.
“This idea came about after I gave birth to my daughter,” says Dr. Whitehead. “I had a lot of time while breastfeeding her to think about what I was doing, and I realized that I didn’t really know very much about breast milk or why it was so good for my baby. When I turned to the scientific literature to try to find answers, I realized that very few answers were out there.”
Dr. Whitehead found that there are many living human cells in breast milk—on the order of a million human cells per milliliter of breast milk. When infants drink breast milk, they consume their mothers’ human cells with it.
These cells have a remarkable property: they can get out of the gastrointestinal tract and functionally integrate into the infant’s tissue. The mother’s cells might enter the liver, for example, attaching there and growing amongst the baby’s own liver cells. These cells can live in the baby long into adulthood.
“This is an absolutely amazing phenomenon,” says Dr. Whitehead. “As drug delivery scientists, we spend a lot of time in my research group trying to think about how to transport proteins and other therapeutic molecules out of the gastrointestinal tract. Living human cells are an order of magnitude bigger than that, and yet, they have the remarkable ability to get out of the GI tract and into the infant’s body. This is amazing—and nobody understands how or why it’s happening.”
In addition to oral drug delivery, Dr. Whitehead’s lab also works on RNA delivery and gene therapy. Dr. Whitehead believes that, if her lab can figure out how these cells are achieving this transport through the body, they might be able to use the cells as drug delivery vehicles for some therapeutic purpose that has never been seen before.
Dr. Whitehead’s long-term vision is to isolate the cells from mother’s pumped milk and genetically engineer them in a dish. From there, the lab would put the genetically engineered cells back into the milk for the baby to consume. By taking the cells in the mother’s milk and isolating them, Dr. Whitehead’s lab could genetically engineer the cells to perform non-invasive therapeutic functions to treat sick babies.
For example, Dr. Whitehead’s lab might be able to task immune cells to help deliver oral vaccinations for babies, instead of taking the babies for shots every few months. Other examples may include tolerizing infants to allergens, such as peanuts, or treating babies with spina bifida, enterocolitis, or other types of genetic disorders.
“This idea is an example of why diversity in science and engineering is important. It was only having gone through the specific experience of childbirth and breastfeeding that allowed me to identify this underdeveloped area,” says Dr. Whitehead. “Our approach will be non-invasive both for the mother and the baby, and with this project we might be able to treat babies in ways we’ve only really dreamed of before.”
Dr. Whitehead’s award was possible, in part, because she had some preliminary data to support her grant proposal. It can be difficult to find funding sources to produce preliminary data in the lab for new ideas, and without initial funding, new ideas have nowhere to go. Dr. Whitehead’s preliminary research was funded by Chemical Engineering alumni donor Jon Saxe and his wife Myrna Marshall.
Manipulating the Skin with Microneedles to Fight Disease
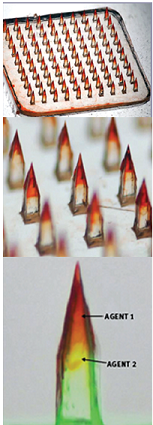
Gavin Jenkins, in his recent article for PittMed, reports on the work of McGowan Institute for Regenerative Medicine affiliated faculty member Louis Falo, Jr., MD, PhD, Professor and Chairman of the Department of Dermatology at the University of Pittsburgh School of Medicine. Seven years ago, and inspired by the history of scratch vaccinations, Dr. Falo began researching microneedle array technology, which delivers medicine through the skin. Afterall, our skin is our biggest organ, and our first line of defense against viruses and other pathogens we come across.
“The control of the body’s entire immune response can probably be achieved by manipulating the skin,” Dr. Falo says. “I have always tried to manipulate immunity by manipulating skin immunity.”
And now his work has led to what is probably the world’s first study examining the effectiveness of microneedles on skin cancer.
A phase I clinical trial is testing proper dosages for patients with cutaneous T-cell lymphoma. It’s still early in the process, but if microneedle therapy pans out, it could change how skin cancer is treated. No more operations. No chemotherapy injections. Just a Band-Aid covering a fingertip-size patch with tiny medication-infused needles.
Microneedles, Dr. Falo says, are better than intradermal injections, which introduce a drug to the dermis (the thick layer of “living tissue” under the epidermis, the outer skin layer). Intradermal injections are hard to reproduce. Microneedles, on the other hand, deliver the same results each time, and they inject medicine directly to both the dermis and epidermis.
The immune system is more efficient in the skin than in other tissues. Dr. Falo has found that immunizations delivered directly to the skin result in stronger and longer-lasting immunizations than those delivered below the skin or into muscle.
When doctors administer chemotherapies through an IV or injection, they destroy rapidly dividing cells faster than other cells. But then the toxins flow throughout the body, producing the side effects of hair loss, nausea, and fatigue.
Dr. Falo believes microneedles can deliver a lower dose of chemotherapy medication straight to squamous cell carcinomas, basal cell carcinomas, and cutaneous lymphomas. The drug’s high concentration to one area would cut down on, and possibly prevent, negative side effects.
“It enters cancer cells in the skin where it is delivered and essentially doesn’t go anywhere else,” Dr. Falo says.
Microneedles might not be there yet, but Dr. Falo says that the field is gaining momentum. And his team also is devoted to reversing autoimmunity with microneedles. In this context, microneedles are a tool for introducing changes to the microenvironment. Down the road, he envisions help for patients with psoriasis, eczema, and contact dermatitis (like poison ivy).
Illustration: Microneedle arrays are fingertip-size patches that deliver drugs right to the skin. The needles can contain multiple adjuvants that stimulate the skin’s immune response. Falo Lab.
Treating “Pre-Cavities”

In 2017, the University of Pittsburgh School of Dental Medicine received an $11.7 million grant from the National Institute of Dental and Craniofacial Research (NIDCR) to establish a resource center dedicated to advancing therapies for regenerating damaged dental, oral, and craniofacial tissues. Officially named the Michigan-Pittsburgh-Wyss Regenerative Medicine (MPWRM) Resource Center: Supporting Regenerative Medicine in Dental, Oral, and Craniofacial Technologies, Pitt established the center in partnership with the University of Michigan and Harvard University as part of the NIDCR’s Dental, Oral, and Craniofacial Tissue Regeneration Consortium (DOCTRC). The goal of the consortium is to guide new therapies from the research stages through preclinical studies and into human clinical trials.
Charles Sfeir, DDS, PhD, associate dean for research and director of Pitt’s School of Dental Medicine Center for Craniofacial Regeneration, and William Wagner, PhD, director of the McGowan Institute for Regenerative Medicine and Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh, are the principal investigators at the MPWRM Resource Center in Pittsburgh.
GreenMark Biomedical Inc. announced it secured funding for its patented dental technology that identifies ‘pre-cavities’ which can be treated non-invasively before becoming cavities. This will have major implications for treatment of tooth decay prior to the need for surgical treatment, resulting in improved oral health outcomes for patients.
GreenMark along with its collaborators at the University of Michigan (U-M) was awarded $110,000 from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH) through the MPWRM Resource Center. This grant will assist GreenMark in its development of a non-invasive treatment product aimed at preventing fully developed cavities.
The company’s project, “Targeted Remineralization Treatment Using Mineral Loaded Starch Nanoparticles,” has been assigned a team of 12 experts from multiple leading resources in dentistry, including the U-M School of Dentistry, University of Pittsburgh’s McGowan Institute & Center for Craniofacial Regeneration, and Harvard University’s Wyss Institute to provide technical advice and support.
According to GreenMark’s Chairman and CEO Steven Bloembergen, PhD, “The NIH support through MPWRM provides additional financial resources and compliments the team we previously assembled for our dental diagnostic product. It is a tremendous vote of confidence for our non-invasive treatment strategy.”
CoreValve U.S. Pivotal High-Risk Trial: 5-Year Durability Data

Medtronic, a global leader in heart valve therapies, recently announced new data presented at the 30th Transcatheter Cardiovascular Therapeutics (TCT) conference, the annual scientific symposium of the Cardiovascular Research Foundation. During the meeting, investigators—including McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Gleason, MD, the Ronald V. Pellegrini Endowed Professor of Cardiothoracic Surgery and Chief, Division of Cardiac Surgery of the University of Pittsburgh School of Medicine, Co-Director of the Heart and Vascular Institute at UPMC, Director of the Center for Thoracic Aortic Disease, Co-Director of the Center for Heart Valve Disease, and member of the Center for Vascular Remodeling and Regeneration at Pitt—presented the longest-term data to-date from the CoreValve U.S. Pivotal Trial
The longest-term follow-up data ever presented from the randomized trial showed that patients implanted with Medtronic’s CoreValve(TM) System experienced excellent valve durability out to five years with low severe hemodynamic structural valve deterioration (0.8 percent) compared to patients who received a surgical aortic valve replacement (SAVR) (1.8 percent). Of the 750 patients (391 transcatheter aortic valve replacement (TAVR); 359 SAVR) followed out to five years, patients treated with the CoreValve System showed similar outcomes compared to surgery for the primary endpoint of all-cause mortality 55.3 percent versus 55.4 percent; p=0.50). Additionally, rates were similar for major stroke (12.3 percent versus 13.2 percent; p=0.49) at five years.
“These were early CoreValve patients, some of the first patients to receive the therapy, and it’s reassuring to see that the CoreValve System has proven to be durable out to five years,” said Dr. Gleason, who presented the data at the meeting. “As the technology and heart team experience continues to improve, this longer-term follow-up data is an encouraging indicator for TAVR patients in the future. Earlier concerns regarding the durability of TAVR are certainly tempered by these mid-term data.”
Studying Ferroptosis

In her report, Elaine Vitone, PittMed, writes of the numerous research efforts of Pitt investigators that are illuminating a kind of cell death called ferroptosis, which likely plays a role in many acute and chronic diseases. Ferroptosis is a type of programmed cell death dependent on iron and characterized by the accumulation of lipid peroxides and is genetically and biochemically distinct from other forms of regulated cell death such as apoptosis.
Ferroptosis is a research focus in the lab of McGowan Institute for Regenerative Medicine affiliated faculty member Valerian Kagan, PhD, DSc, Professor and Vice-Chairman in the Department of Environmental and Occupational Health as well as a Professor in the Department of Pharmacology and Chemical Biology, the Department of Radiation Oncology, and the Department of Chemistry at the University of Pittsburgh. He is also the Director of the Center for Free Radical and Antioxidant Health. Within the massive field of programmed cell death, ferroptosis is exploding. Pitt researchers are among those leading such efforts, having developed a new technology for the study of ferroptosis—Pitt is one of the few places in the world with this capability.
Dr. Kagan has collaborated with many labs at Pitt to better understand “the reason” for ferroptosis—exactly what biomolecular line is crossed, how that signal is communicated within and between cells, which molecules pull the trigger, and how. Combining clinical observations from multiple fields of medicine, along with biochemistry, molecular biology, structural biology, and computational biology, they’ve uncovered new insights with potential relevance to a number of diseases. They hope to find ways to stem ferroptosis when it contributes to the degradation of tissue, as it does in brain trauma, asthma, kidney disease, and more—and, in the case of cancer, to better urge ferroptosis into action.
Dr. Kagan and his Pitt colleagues work together with other labs in the U.S. and plans for an international collaboration are in the works. In time, the Pitt team’s bicontinental efforts could grow to a global ferroptosis force to be reckoned with.
Change in Transplant Rules Has Chance to Save Lives

Before the United Network for Organ Sharing, or UNOS, made the heart and lung transplantation organ allocation change in November 2017, the United States was divided into 58 local donor service areas. People on the heart and lung transplant list were first matched with donors within their service areas, even if another potential match was closer geographically.
McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan D’Cunha, MD, PhD, FACS, Chief of Lung Transplantation at UPMC said, “Prior to Thanksgiving of last year we had donor service areas where the lungs were allocated first to the area based on severity of illness. And then they went out to the national pool after that, after the local centers passed on the lungs. So, what happened was there was actually a lawsuit last fall around the time of Thanksgiving where one of these lines that was drawn, there was a sick patient across the border.”
This change is making a difference for many patients: “Prior to Thanksgiving, patients on our wait list would wait somewhere between 175 and 200 days. And now you’re transplanting people within 25 to 35 days currently,” said Dr. D’Cunha.
Many people with lung disease, cystic fibrosis is an example, face the possibility of a lung transplant. Lung transplantation can extend and improve the quality of life, but it involves an extensive evaluation process and a commitment to living the lifestyle required to keep your new lungs healthy. Donors may have previously agreed, in the case of their death, to give their healthy lungs to people who need them. However, it is vital that a person who wishes to donate their organs at the time of death have a discussion with their families of their expressed wish. If a person dies unexpectedly and has not previously indicated whether his or her organs may be donated to those in need, the family may give doctors permission to donate their loved one’s lungs.
Researchers Report on Promising Liver Cell Transplantation Technologies

The need for liver transplantation is expected to rise by 23 percent over the next two decades, but the supply of transplantable livers is inadequate today, leaving patients in need of liver transplantation with increasingly longer waiting periods and worse prospects for survival. To save the lives of more patients suffering from liver diseases, patients, their families, doctors, and researchers are all looking toward improving liver cell transplantation using autologous (patient-donated) liver cells.
“Liver cell therapy has been under intensive investigation for decades and is a promising alternative to liver transplantation,” said study co-author Alejandro Soto-Gutierrez, MD, PhD, an Associate Professor of the University of Pittsburgh Department of Pathology and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, Children’s Hospital of Pittsburgh of UPMC, and the Thomas E. Starzl Transplantation Institute. “However, liver cell transplantation has, to date, not been reliable enough to replace liver transplantation.”
According to the authors, significant barriers limits the broader implementation of liver cell therapy. First, sources are lacking for liver cells (hepatocytes) appropriate for transplantation; second, the inability to monitor the function or rejection of transplanted cells is a serious hurdle.
“The ability to use cells induced from the kinds of cells able to differentiate into a great variety of cell types will dramatically facilitate cell therapies for patients with some liver disorders,” said Dr. Soto-Gutierrez. “Our objective in this paper was to evaluate the current state of efforts aimed at developing patient self-donated liver cells for transplantation.”
The authors investigated several recent technological advancements that may change the cell therapy landscape. The most promising advancements they evaluated included:
- the promise found in the ability to generate hepatocyte-like cells (HLCs) from cells called pluripotent stem cells (iPSCs);
- the ability to use gene ‘editing,’ using the CRISPER/CAS9 system (CRISPR is an abbreviation of Clustered Regularly Interspaced Short Palindromic Repeats) to either correct loss of gene function or deficient gene expression; and
- using living animal’s bodies (in vivo) as ‘bioreactors’ to make and mature large numbers of functional human hepatocytes for transplantation.
However, each process and technology has its advantages and limitations, and each requires more research and development.
“Unfortunately, stem cell-derived HLCs do not possess all of the functions of mature hepatocytes,” explained Dr. Soto-Gutierrez. “Too, with gene editing, there is risk for off-target gene editing in non-target genes or in tissues. That risk can be minimized in the near future. Third, larger, more costly mammals are needed to produce human hepatocytes.”
“Successful liver cell transplantation will require optimizing best cell functions, overcoming limitations to cell numbers and safety, as well as a number of other challenges that currently limit the success of the three approaches we discussed in our paper,” concluded the authors. “Collaboration among scientists, clinicians and industry will be critical for generating new autologous stem cell-based therapies to treat liver diseases.”
“The authors have summarized these promising technologies with scientific rigor and have apprised us of where we stand with regard to the future of regenerative therapy for liver diseases,” said Prof. Frederic Lemaigre of the Université Catholique de Louvain, de Duve Institute, in Brussels, Belgium. “They have clarified which issues have been solved and have, without making unfounded promises, reminded us of challenges remaining.”
Integrated Clinical and Research Systems for Diabetic Foot Wound Care
From the desk of McGowan Institute for Regenerative Medicine faculty member J. Peter Rubin, MD, UPMC Endowed Professor and Chair of Plastic Surgery, Director, UPMC Wound Healing Services, Professor of Bioengineering, University of Pittsburgh:
I am pleased to report that our proposal to the National Institutes of Health (NIH), 1U01 DK 119102 – 01, “Integrated Clinical and Research Systems for Diabetic Foot Wound Care,” has been awarded by the National Institute of Diabetes and Digestive and Kidney Diseases. With this 4-year U01 award, we join a select group of academic institutions in a clinical research consortium that aims to study biomarkers for diabetic foot ulcer healing. This award leverages the clinical expertise of our new UPMC Wound Healing Service line and the scientific power of the University of Pittsburgh.
Diabetes is a common, complex, and costly disease affecting 9.4% (30.3 millions) of Americans. It remains the 7th leading cause of death in the United States, contributing to over 250,000 deaths annually. Diabetic foot ulcers (DFU) are the most frequently recognized complication in diabetics with an incidence of 6% in the diabetic global population, 6% among Medicare diabetic beneficiaries, 5% among diabetic U.S. veterans, and a lifetime incidence of foot ulcers between 19% and 34% in all diabetics. The natural history of a diabetes-related foot ulcer is devastating. More than half of ulcers become infected and approximately 20% of moderate or severe diabetic foot infections lead to amputation. Mortality after DFU-related amputations is greater than 70% at 5 years, which is 2.5 times higher than in diabetic patients without a foot ulcer.
Our wound healing clinical research team will be embedded within 3 sites in the UPMC Wound Healing Services network and will systematically collect and analyze data that can be potentially useful for predicting the severity and prognosis of diabetic foot ulcers. The data will be correlated with the clinical course of healing, and rigorous, evidence-based clinical pathways followed in order to standardize treatment. Our clinical providers and consultants across numerous disciplines will help evaluate and refine the treatment pathways. Importantly, we will address the major challenges of diabetic foot ulcer clinical research through the seamless integration of wound center clinical operations and research operations. We will be implementing research recruiting and research conduct practices that allow us to learn from our diverse patient population while maintaining high clinic efficiency and outstanding care. This is a tremendous team effort, with a multidisciplinary approach and unique partnership involving the clinical care providers, the researchers, and our data systems experts.
This NIH award helps put our new service line on a path to important contributions in the field of wound healing and I sincerely thank all of you for your collaboration. It is only with your expertise that we can establish this unique effort and evolve new standards.
J. Peter Rubin
McGowan Institute for Regenerative Medicine affiliated faculty members Lauren Kokai, PhD, research faculty member in the Department of Plastic Surgery, School of Medicine, and co-director of the Adipose Stem Cell Center at the University of Pittsburgh, and MaCalus Hogan, MD, Vice Chair of Education and Residency Program Director in the Department of Orthopaedic Surgery at UPMC, and Assistant Professor in the Departments of Orthopaedic Surgery and Bioengineering at Pitt, are members of the research team working on this project.
AWARDS AND RECOGNITION
Dr. Antonio D’Amore Wins ISSNAF Franco Strazzabosco Award for Young Engineers

In pursuing its mission, the Italian Scientists and Scholars of North America Foundation (ISSNAF) grants specific focus to early stage investigators working in North America whose commitment to their discipline of study is innovative, impactful, and honors their country of origin.
McGowan Institute for Regenerative Medicine affiliated faculty member Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh, was selected as this year’s recipient of the ISSNAF Franco Strazzabosco Award for Young Engineers. This award was established by the Strazzabosco family in 2013 in memory of their late father, Franco Strazzabosco. It is a tribute to the entrepreneurial courage of Italian engineers, who strive in applying scientific discoveries to public advantage.
On November 8, 2018, at the Italian Embassy in Washington, DC, Dr. D’Amore will be honored with the Medaglia di Rappresentanza del Presidente della Repubblica Italiana and receive a prize of $3,000.
Dr. D’Amore’s research seeks to couple a mechanistic understanding of the relationship between scaffolds micro-structure, mechanics, and endogenous tissue growth with the development of novel biomaterials for tissue engineering strategies. The focus of his research is upon unmet clinical needs in cardiovascular diseases. Recent areas of interest include: quantitative histology and biomaterials micro-structure image-based analysis, structural modeling strategies to guide tissue engineering scaffold fabrication, development of cardiac restrain devices, vascular grafts and engineered heart valves. Dr. D’Amore’s current project funding comes from the National Institutes of Health, RiMED Foundation, The Wallace H. Coulter Foundation, and the University of Pittsburgh.
Dr. D’Amore thanked William Wagner, PhD, Director of the McGowan Institute, for his “invaluable mentorship over the years. A personal success never belongs to one person only,” said Dr. D’Amore.
Graduated in Electro-technical Engineering from Universita’ di Padova in 1953, Franco Strazzabosco ran a successful career in the field of hydroelectric power with Montecatini, Gruppo SNIA, Gas Metano Camerini, and Metano BSP. As CEO and main shareholder of C.I.GAS S.p.A., Franco Strazzabosco contributed to Italian energy development, supplying services which improved people’s quality of life and industrial operational capabilities. His entrepreneurial initiative marked the historic time of Italy’s gas grid development thanks to the positive interaction between private entrepreneurship and local Government.
Congratulations, Dr. D’Amore!
PInCh 2018: What Is Your Idea to Improve Human Performance?

A total of $475,000 in prizes were awarded at the Pitt Innovation Challenge (PInCh®) final event, in which teams competed with projects aimed at improving human performance, which was defined broadly as benefitting physiological or psychological functioning of healthy people or those with chronic disease.
The challenge, which is in its fifth year, was sponsored by the University of Pittsburgh’s Clinical and Translational Science Institute (CTSI). Three of the eight $25,000 project finalists who competed in a poster session involved affiliated faculty members from the McGowan Institute for Regenerative Medicine on the winning teams. These awarded projects include:
motionSleeve
Team Leaders: Nitin Sharma
Team Members: Ashwin Iyer, Kang Kim, and Thomas Hinds
Description: A wearable device that detects muscle tremors in real-time using ultrasound sensors, then provides muscle stimulation to alleviate the tremor.
PopSole
Team Leaders: Jeffrey Gusenoff and Beth Gusenoff
Team Members: MaCalus Hogan, Kentaro Onishi, and William Anderst
Description: A novel insole designed to reduce post-operative pain, maximize healing, and encourage early ambulation and return to function.
Quantitative Ultrasound to Prevent Tendon Injury (QUPTI)
Team Leaders: Richard Debski
Team Members: Kang Kim, Volker Musahl, and Gerald Ferrar
Description: A novel ultrasound technique to instantly assess location-specific tendon damage and weakening, informing instantaneous clinical and sports decisions.
“The PInCh competition is designed to provide teams of researchers, innovators, clinicians and community collaborators with a venue to create novel solutions to important clinical and public health problems,” said CTSI director Steven Reis, MD, who also is associate vice chancellor for clinical research, health sciences, and a professor of medicine at Pitt.
HERL Receives VA Merit Review Grants

The U.S. Department of Veterans Affairs (VA) has awarded three VA Merit Review Grants to the Human Engineering and Research Laboratory (HERL). The mission of HERL is to continuously improve the mobility and function of people with disabilities through advanced engineering in clinical research and medical rehabilitation.
Two of the projects include McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, FISA & Paralyzed Veterans of America (PVA) Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine & Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, and Founding Director and VA Senior Research Career Scientist of HERL, as principal investigator and co-principal investigator.
The 4-year R01 awards are:
- RX002753-01A2, “Clinical Evaluation of Pneumatic Technology for Powered Mobility Devices” – Brad Dicianno, MD, and Rory Cooper, PhD
- RX002794-01A2, “Development of TransKinect: A Clinically Robust System for Transfer Assessment” – Alicia Koontz, PhD, RET
- RX002755-01A2, “Powered Person Transfer System” – Rory Cooper, PhD
Congratulations, all!
Dr. Warren Ruder Receives NIH Director’s Award

McGowan Institute for Regenerative Medicine affiliated faculty member Warren Ruder, PhD, assistant professor in the Department of Bioengineering at the University of Pittsburgh, receives prestigious honor from the NIH High-Risk, High-Reward Research Program. Dr. Ruder joins Peter Strick, PhD, scientific director of the University of Pittsburgh Brain Institute and distinguished professor and chair of neurobiology, and Erik Wright, PhD, an assistant professor of biomedical informatics, as a recipient of this year’s recognition.
The NIH High-Risk, High-Reward Research program accelerates scientific discovery by supporting creative, trailblazing ideas in clinical and basic biomedical science that may struggle under the conventional funding mechanism but could have a transformative impact in addressing important challenges in medicine.
Dr. Ruder was awarded an NIH Director’s New Innovator Award, which is given to support “exceptionally creative new investigators who propose highly innovative projects,” according to the NIH.
Dr. Ruder’s research group works at the interface of biology and engineering to create new biomimetic systems that both provide insight into biological phenomena and serve as platform technologies for future medical applications. This award will support the development of engineered cells that can be activated by high magnetic field gradients.
“I am proud of Warren for being the second Swanson School faculty member to win this extremely competitive and prestigious award,” said James Martin, PhD, U.S. Steel Dean of Engineering. “His research underscores the Swanson School’s goal to produce creative and innovative research that has the potential to transform our world.”
“This program supports exceptionally innovative researchers who have the potential to transform the biomedical field,” said NIH Director Francis Collins, MD, PhD. “I am confident this new cohort will revolutionize our approaches to biomedical research through their groundbreaking work.”
Illustration: University of Pittsburgh Swanson School of Engineering.
Bridgeside Research Forum Spring 2018 Travel Award Winners Announced

The Bridgeside Research Forum is a seminar series given by postdocs and graduate students every Friday during the University of Pittsburgh academic year.
The Bridgeside Research Forum Spring 2018 Travel Awards for best presentation were recently announced and the winners are as follows (pictured left to right):
- Caylin Winchell (Flynn Lab, Department of Microbiology and Molecular Genetics)
- Patrick Wilkinson (Lab of Andrew Duncan, PhD, McGowan Institute for Regenerative Medicine)
- Chelsea Vadnie (McClung Lab, Department of Psychiatry)
- Leonid Emerel (Lab of Thomas Gleason, MD, McGowan Institute for Regenerative Medicine)
Congratulations, all!
McGowan Institute Laboratory Building Recognized During GBA Silver Anniversary Gala

Pittsburgh’s Green Building Alliance (GBA) celebrated its silver anniversary at the Emerald Evening Fundraising gala, an annual event to recognize local construction projects that emphasize green, sustainable design. This year’s ceremony was especially auspicious.
For a quarter of a century, the GBA has provided advocacy, networking resources, and technical support to hundreds of construction and rehabilitation projects across the state.
“The 25th anniversary of Green Building Alliance is an opportunity to reflect on the region’s outstanding achievements in sustainability while celebrating the incredible innovation underway,” says GBA Executive Director Jenna Cramer. “The sustainability movement is indelibly linked to Pittsburgh’s transformation, and we are honored to celebrate the people who continue to create places and a region where everyone can thrive.”
To mark the milestone, the GBA recognized 25 innovative green structures built in the last 25 years. The University of Pittsburgh’s McGowan Institute for Regenerative Medicine laboratory building at 3025 E. Carson St. in Pittsburgh’s South Side was an honoree: Perhaps the most sci-fi space on the list, researchers at the McGowan Institute are making stunning strides in the field of artificial organs within this green building. In 2005, Pitt announced that this was the first university building in Pennsylvania to earn LEED (Leadership in Energy and Environmental Design) Gold status.
A sustainable or “green” building is one that emphasizes an integrated approach to building design, engineering, material selection, energy efficiency, lighting, furnishings, technology, building operations, maintenance, and waste management strategies. It should be practical, economical and make a natural connection to both the community and the land.
And finally, an environmentally sensitive building should be beautiful, warm, and a pleasant place to work and learn…the goal for the McGowan Institute building. The building is a 45,200-square-foot, two-story building approximately three miles east of downtown Pittsburgh on a former LTV steel site. The building holds office and laboratory space for more than 100 scientists, researchers, and staff developing such cutting-edge medical breakthroughs as artificial hearts and lungs and other life-saving devices. The McGowan Laboratory Building helped launch the economic revitalization of the East Carson corridor of Pittsburgh’s historic South Side.
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#189 –– Dr. Robert Guldberg discusses the new Phil and Penny Knight Campus for Accelerating Scientific Impact at the University of Oregon. He also discusses his role with the Journal of Immunology and Regenerative Medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Agarwal N, Popovic B, Martucci NJ, Fraunhoffer NA, Soto-Gutierrez A.
Title: Biofabrication of Autologous Human Hepatocytes for Transplantation: How do we get there?
Summary: Directed differentiation of hepatocytes from induced pluripotent stem cells (iPSCs) holds promise as source material for treating some liver disorders. The unlimited availability of perfectly differentiated iPSC-derived hepatocytes will dramatically facilitate cell therapies. While systems to manufacture large quantities of iPSCs-derived cells have been developed, we have been unable to generate and maintain stable and mature adult liver cells ex vivo. This short review highlights important challenges and possible solutions to the current state of hepatocyte biofabrication for cellular therapies to treat liver diseases. Successful cell transplantation will require optimizing the best cell function, overcoming limitations to cell numbers and safety, as well as a number of other challenges. Collaboration among scientists, clinicians, and industry is critical for generating new autologous stem cell-based therapies to treat liver diseases.
Source: Gene Expr. 2018 Aug 24. [Epub ahead of print]
GRANT OF THE MONTH
PI: William R, Wagner, PhD Co-investigator, Donald Taylor, PhD, MBA Co-investigator
Title: Philadelphia-Pittsburgh Pediatric Medical Device Consortium
Description: The PPDC will serve as a national resource for pediatric device development. The consortium will provide a platform of experienced regulatory, business planning, and device development services to help foster and guide the advancement of medical devices for pediatric patients. The consortium will provide expert advising and support services to innovators of pediatric medical devices and this work will focus on the total product life cycle for pediatric medical devices and not just getting the device to market. We intend to use the majority of funding received through this grant to encourage innovation, mentor projects, advise on potential funding resources, facilitate the connection of innovators with physicians and business planners, and provide advice to inventors on matters such as how to meet regulatory requirements.
Source: U.S. Food and Drug Administration
Amount: $1,000,000
