May 2019 | VOL. 18, NO. 05| www.McGowan.pitt.edu
Clinical Trials Moving Forward for ALung Technologies, Inc.
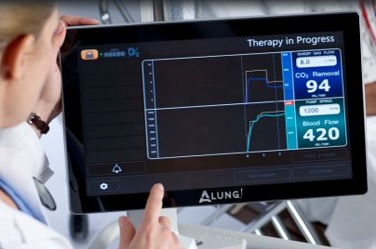
Technology developed in the McGowan Institute Medical Devices Lab under the leadership of William Federspiel, PhD, William Kepler Whiteford Professor of Bioengineering, Chemical Engineering, and Critical Care Medicine has been incorporated into products for clinical use by ALung, a University of Pittsburgh spinout company. Dr. Federspiel is the Head of the Scientific Advisory Board and co-founder of ALung Technologies, Inc. The ALung devices are now being evaluated in several clinical trials. ALung is the leading provider of low-flow extracorporeal carbon dioxide removal (ECCO2R) technologies for treating patients with acute respiratory failure.
Currently, ALung is working on major milestones in its clinical trial programs of the Hemolung Respiratory Assist System (RAS). The Hemolung RAS was born from research efforts and technologies developed at the McGowan Institute.
In the United States, ALung’s VENT-AVOID Trial of the Hemolung RAS is ongoing. In the United Kingdom, the REST Trial, a landmark pivotal study of the Hemolung RAS in patients with acute respiratory distress syndrome (ARDS), is moving ahead as well.
The trials in the U.K. are designed to test 1,120 patients, while the U.S. trials may have up to 800 participants depending on the success of early patients. So far, 36 are enrolled in the U.S. and 360 across the U.K. Peter DeComo, ALung’s Chairman and CEO, estimates that the trials will carry on for another two or three years.
COPD affects 30 million Americans and is the third leading cause of death in the United States behind cancer and heart disease. Acute exacerbations, defined as a sudden worsening of COPD symptoms, are a major cause of morbidity and mortality in COPD patients. The VENT-AVOID Trial is the world’s first pivotal study of extracorporeal carbon dioxide removal in the AE-COPD population. The study aims to validate the safety and efficacy of the Hemolung RAS for COPD patients experiencing acute exacerbations requiring ventilatory support.
Illustration: ALung Technologies, Inc.
RESOURCES AT THE MCGOWAN INSTITUTE
June Histology Special
Elastin is a protein in connective tissue that allows many tissues in the body to resume their shape after stretching or contracting. Elastin helps skin to return to its original position when it is poked or pinched.
Elastin serves an important function in arteries as a medium for pressure wave propagation to help blood flow and is particularly abundant in large elastic blood vessels such as the aorta. Elastin is also very important in the lungs, elastic ligaments, the skin, and the bladder, elastic cartilage. It is present in all vertebrates above the jawless fish
Verhoeff’s stain forms a variety of cationic, anionic and non-ionic bonds with elastin, the main constituent of elastic fiber tissue. Elastin has a strong affinity for the iron-hematoxylin complex formed by the reagents in the stain and will hence retain dye longer than other tissue elements, which allows elastin to remain stained while other tissue elements are decolorized. Elastic fibers and cell nuclei are stained black, collagen fibers are stained red, and other tissue elements including cytoplasm are stained yellow.
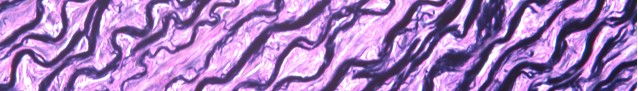
You’ll receive 25% off your VVG stain every day in June. Contact Lori at the McGowan Core Histology Lab and ask about our VVG specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology work with the quickest turn-around time.
SCIENTIFIC ADVANCES
Beckman Coulter’s Early Sepsis Indicator Receives 510(k) Clearance from the U.S. FDA

A major milestone on its strategic mission to lead in sepsis diagnostics, Beckman Coulter recently announced that its Early Sepsis Indicator has received 510(k) clearance from the U.S. Food and Drug Administration. Sepsis is a global healthcare crisis that affects more than 30 million people worldwide. The Early Sepsis Indicator is a first-of-its-kind, hematology-based cellular biomarker that is designed to help emergency department physicians identify patients with sepsis or at increased risk of developing sepsis.
As part of the pivotal clinical trial for the Early Sepsis Indicator, findings showed that Beckman Coulter’s unique monocyte distribution width (MDW) biomarker best discriminated sepsis from all other conditions when combined with the current standard of care. McGowan Institute for Regenerative Medicine affiliated faculty member Derek Angus, MD, MPH, Chair of the Department of Critical Care Medicine of both the University of Pittsburgh School of Medicine and the UPMC Healthcare System, a collaborator in the study, said that the Early Sepsis Indicator is “a novel feature in that it is exploiting the way in which white blood cell counts are already calculated.”
The Early Sepsis Indicator is automatically reported as part of a routine complete blood count (CBC) with differential for adult emergency department patients. A positive Early Sepsis Indicator result signals a higher probability of sepsis, enabling physicians to initiate lifesaving treatments faster. Conversely, a negative reading indicates a lower probability of sepsis. Compared to reviewing white blood cell count alone, the Early Sepsis Indicator strengthens a clinician’s suspicion of sepsis by 43% and, together with clinical signs and symptoms, improves their confidence in helping to rule out sepsis by 63%.
Researchers Receive a $2.5M NIH Award to Create an Improved Repair Device for POP

Pelvic organ prolapse (POP) is a condition where the organs in the pelvis push against the vagina, creating a “bulge” that can extend outside of the body. It results from a weakening of the muscles and tissues that help support the pelvic organs. This disorder affects many women, but surgical treatments with polypropylene mesh – devices initially designed for hernia repairs, not vaginal use – often result in complications.
Researchers from the University of Pittsburgh will use a five-year, $2,500,000 award from the National Institutes of Health to create a novel repair device designed for the vagina that may improve outcomes in POP surgery. McGowan Institute for Regenerative Medicine affiliated faculty member Pamela Moalli, MD, PhD, professor of obstetrics, gynecology, and reproductive sciences at Pitt and pelvic reconstructive surgeon at UPMC Magee-Womens Hospital, and Steven Abramowitch, PhD, associate professor of bioengineering in the Swanson School of Engineering, will lead this effort.
Drs. Abramowitch and Moalli co-direct the Center for Interdisciplinary Research in Female Pelvic Health (CIRPH) where they focus on the impact of pregnancy, delivery, and other life-changing events on the structural integrity of the pelvic floor. They aim to develop preventative treatment options for POP and more effective patient-specific treatments.
“The purpose of this project is to design and develop novel solutions for POP repair since past materials were never specifically developed for the functional and material properties of the vagina,” said Dr. Moalli. “Based on our studies, the properties of most meshes are altered following implantation with tensioning and loading, which in some cases can lead to suboptimal outcomes. Ultimately, this funding mechanism will allow us to design a device with properties that more closely mimic the native properties of the vagina and its supportive tissues. We believe this approach will create a more favorable biological response compared to current mesh products.”
“Our group will evaluate the use of elastomeric polymers whose inherent stiffness is similar to that of the vagina but are also tough enough to meet the physiologic loading demands within the pelvis,” explained Dr. Abramowitch. “The designed solutions will contain pore geometries that provide the device with counterintuitive mechanical behaviors, such as the ability to expand rather than contract when you pull on it. We believe that this will allow for better integration between our device and the patient’s tissue.”
Drs. Abramowitch and Moalli hope that their novel device, designed specifically for the vagina, will significantly improve the outcomes of prolapse surgeries while minimizing complications.
“Issues that negatively impact the quality of life and are specific to women often do not get the attention that they deserve in research,” said Dr. Moalli. “This is an opportunity to develop solutions for women that are designed based on an understanding of the uniqueness of female anatomy and biology.”
Stimulation Remodels the Epileptic Brain to Prevent Seizures

Responsive neurostimulation (RNS) treats epilepsy by detecting seizures and intervening with a jolt of electric current. Over time, most patients find their seizures become fewer and further between. Now, for the first time, researchers at the University of Pittsburgh School of Medicine and UPMC—including McGowan Institute for Regenerative Medicine affiliated faculty member R. Mark Richardson, MD, PhD, associate professor of neurological surgery at Pitt’s School of Medicine and director of epilepsy and movement disorders surgery at UPMC—have a better understanding of why this happens.
As reported in JAMA Neurology, new evidence suggests RNS can remodel the brain to be less susceptible to seizures. Using patients’ brain signatures as a guide, the researchers hope to rapidly optimize the use of neurostimulation to help more people achieve seizure reduction.
“Right now, in epilepsy patients treated with responsive neurostimulation, you just have to wait and see whether their seizure frequency goes down,” said Dr. Richardson. “We don’t have a great way to predict who will respond. But there’s so much more data recorded on these RNS devices than we currently have the ability to analyze.”
In patients implanted with the RNS system, baseline brain activity is recorded for a month to characterize a person’s individual seizure patterns. This information is then used to train the stimulator to automatically respond to a seizure as it’s happening.
What no one has done before, Dr. Richardson said, is to analyze each individual recording of seizure activity captured by the device over time to see what is different about the brain activity of patients who experience a reduction in seizure frequency as a result of neurostimulation.
“I was expecting to find what was traditionally assumed—that the electric pulse acutely stops the seizure,” said lead author Vasileios Kokkinos, PhD, a neurosurgery research instructor at Pitt. “I realized this was not what was happening. I saw some examples of acute stopping of the seizures, but that only accounted for 5% of all seizures. It couldn’t explain the 60–70% of patients who responded to treatment.”
Dr. Kokkinos found that people who ultimately experienced fewer seizures after starting neurostimulation treatment showed progressive reductions in spontaneous hyper-synchronous brain activity, starting as early as two months after the stimulator was first turned on. Dr. Richardson contrasted the faster, objective onset of these brain activity changes against the slower, subjective process of waiting to see symptom improvement in a patient’s self-reported diary of seizure activity.
Dr. Richardson hopes this kind of brain activity analysis will provide quicker feedback during the trial-and-error process of parameter adjustment, so that patients can see the long-term benefits of responsive neurostimulation sooner.
“Our next move is to incorporate what we’ve learned into a formal pipeline for analysis that might allow us in the future to predict which patients are responding before they’re able to report that to us clinically,” Dr. Richardson said.
Additional authors on the study include Nathaniel Sisterson and Thomas Wozny, MD, both from Pitt.
This research was funded by the Walter L. Copeland Fund of the Pittsburgh Foundation and National Institutes of Neurological Disorders and Stroke grant R01 NS110424.
Illustration: McGowan Institute for Regenerative Medicine.
Abstract (Association of closed-loop brain stimulation neurophysiological features with seizure control among patients with focal epilepsy. Vasileios Kokkinos, PhD; Nathaniel D. Sisterson, BA; Thomas A. Wozny, MD; R. Mark Richardson, MD, PhD. JAMA Neurology; published online April 15, 2019.)
FLARE IDE Study Results Published; FLASH Study Continues

Inari Medical, Inc. announced the publication of its 106-patient prospective multicenter FlowTriever Mechanical Pulmonary Embolectomy (FLARE) study for the treatment of intermediate-risk pulmonary embolism (PE). The study was published in JACC: Cardiovascular Interventions. FLARE was conducted under the direction of co-principal investigators, Kenneth Rosenfield, MD, Section Head for Vascular Medicine and Intervention at Massachusetts General Hospital, Boston, and Victor Tapson, MD, Associate Director, Pulmonary and Critical Care Division at Cedars-Sinai Medical Center, Los Angeles.
“With FLARE complete and the data now published, we are excited to collect a larger set of real-world data on FlowTriever via the FLASH registry,” added McGowan Institute for Regenerative Medicine affiliated faculty member Catalin Toma, MD, Interventional Cardiologist, UPMC Presbyterian and principal investigator for FLASH (FlowTriever All-Comer Registry for Patient Safety and Hemodynamics) and co-author on the paper on FLARE. Intermediate and high-risk PE patients treated with FlowTriever will be enrolled in FLASH and followed over the short and intermediate term. Patient enrollment in FLASH began in December 2018.
The FLARE study met both of its primary safety and effectiveness endpoints, showing large and rapid reduction in right heart strain, with no device related major adverse events in the 106 patients enrolled. Just two patients received thrombolytic drugs. The study also showed patients treated with FlowTriever had much shorter ICU and overall length of stay compared to previously published studies in which thrombolytic drugs were used to treat PE. FlowTriever has been used to treat over one thousand PE patients to date and is the only thrombectomy device cleared by FDA for pulmonary embolism.
“The results of this trial open the door to an entirely new approach to the treatment of pulmonary embolism. It is exciting to be able to offer this potentially life-saving therapy to our patients,” said Thomas M. Tu, MD, Director of the Pulmonary Embolism Response Team (PERT) at Baptist Health Louisville.
“Appropriate treatment of PE has been hampered by a paucity of data over the years,” said Bill Hoffman, CEO of Inari. “FLARE and FLASH reflect Inari’s commitment to producing and publishing a robust portfolio of clinical data to help physicians understand which patients benefit most, and in what ways, from interventional treatment for PE.”
Abstract (A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism–the FLARE study. Thomas Tu, Catalin Toma, Victor F. Tapson, Christopher Adams, Wissam A. Jaber, Mitchell Silver, Sameer Khandhar, Rohit Amin, Mitchell Weinberg, Tod Engelhardt, Monica Hunter, David Holmes, Glenn Hoots, Hussam Hamdalla, Robert L. Maholic, Scott M. Lilly, Kenneth Ouriel, Kenneth Rosenfield and for the FLARE Investigators. JACC: Cardiovascular Interventions; Volume 12, Issue 9, May 2019.)
Direct Oxidative Stress Damage Shortens Telomeres
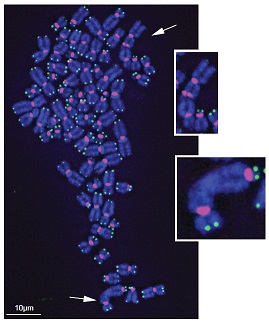
The same sources thought to inflict oxidative stress on cells—pollution, diesel exhaust, smoking and obesity—also are associated with shorter telomeres, the protective tips on the ends of the chromosomal shoelace.
A new study from the University of Pittsburgh, published in Molecular Cell, provides the first smoking gun evidence that oxidative stress acts directly on telomeres to hasten cellular aging. McGowan Institute for Regenerative Medicine affiliated faculty member Simon Watkins, PhD, Founder and Director of the Center for Biologic Imaging at the University of Pittsburgh, a member of the Pittsburgh Cancer Institute, and also a Professor and Vice Chairman within the Department of Cell Biology, is a co-author on the study.
“Telomeres consist of hundreds of guanine bases, which are sinks for oxidation,” said senior author Patricia Opresko, PhD, professor of environmental and occupational health at the Pitt Graduate School of Public Health and UPMC Hillman Cancer Center. “Is it just a coincidence? Or could it be true that oxidizing those guanines in the telomeres is really contributing to shortening?”
To find out for sure, Dr. Opresko needed some way to inflict oxidative stress on telomeres and nowhere else.
So, she enlisted the help of Marcel Bruchez, PhD, professor of biological sciences and chemistry and director of the Molecular Biosensors and Imaging Center at Carnegie Mellon University. Dr. Bruchez developed a method for zeroing in on the telomeres using a special light-activated molecule that latches onto the telomere and delivers localized free radicals—the molecular agent of oxidative stress—on command.
“One of the main challenges to targeting oxidative damage to specific loci in living cells has been achieving precise temporal and dose-control of this damage,” Dr. Bruchez said. “By combining telomere targeting with our optochemogenetic generation of singlet oxygen, we are able to selectively control when and how hard the oxidative stress is applied specifically at the telomere sites.”
The researchers repeatedly exposed cultured cancer cells to this targeted oxidation procedure, mimicking conditions of environmental oxidative stress and inflammation, and, indeed, they saw the telomeres break and shorten with each cell division, despite repair efforts by the telomere lengthening enzyme telomerase.
As the DNA repair machinery tried to fix the broken telomeres, the ends of the chromosomes often fused together, destabilizing the genome and preventing cells from dividing properly.
Whereas telomere shortening spells bad news for healthy cells, Dr. Opresko said, the flipside is that targeting telomeres might offer a way to fight cancer. With short enough telomeres, cancer cells would stop dividing.
“If we can understand what causes telomere shortening and how cells compensate for that,” Dr. Opresko said, “then we’ll be in a better position to design intervention strategies that protect telomeres in healthy cells and target telomeres in cancer cells.”
Illustration: Title: Damaged telomeres get stuck together. Caption: Chronic oxidative damage to telomeres (green) on the ends of chromosomes (blue) causes chromosome fusions (white arrows). Credit: Fouquerel et al. (2019). Mol Cell.
Abstract (Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Elise Fouquerel, Ryan P. Barnes, Shikhar Uttam, Simon C. Watkins, Marcel P. Bruchez, Patricia L. Opresko. Molecular Cell; published: May 14, 2019.)
Efforts Toward Human Performance Optimization

In her PittMed article, Elaine Vitone reviewed human performance optimization efforts by numerous University of Pittsburgh researchers and the results of their studies on the functioning of the human body. Included in her article were highlights from the work of McGowan Institute for Regenerative Medicine affiliated faculty members:
- Ronald Poropatich, MD, Director of the Center for Military Medicine Research (CMMR), Health Sciences and Professor of Medicine in the Division of Pulmonary, Allergy, and Critical Care Medicine at the University of Pittsburgh, and also serves as a Senior Advisor for Telemedicine, University of Pittsburgh Medical Center
- Rory Cooper, PhD, FISA & Paralyzed Veterans of America (PVA) Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, Professor of Bioengineering, Physical Med & Rehab, and Orthopedic Surgery, Founding Director and VA Senior Research Career Scientist of the Human Engineering Research, an Adjunct Professor in the Robotics Institute of Carnegie Mellon University, and PM&R of the Uniformed Services University of Health Sciences
- Freddie Fu, MD, Distinguished Service Professor and the David Silver Professor of Orthopaedic Surgery and Chairman of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine and University of Pittsburgh Medical Center with secondary appointments at Pitt as a Professor of Physical Therapy, School of Health and Rehabilitation Sciences, Professor of Health and Physical Activity, School of Education, and Professor of Mechanical Engineering & Materials Science, Swanson School of Engineering and, since 1986, head team physician for the University of Pittsburgh Department of Athletics
- Bradley Nindl, PhD, Director of the Neuromuscular Research Laboratory/Warrior Human Performance Research Center and Professor in the Department of Sports Medicine in the School of Health and Rehabilitation Sciences at the University of Pittsburgh
Human performance is a crucial concern for the American military, says Dr. Poropatich, who through the CMMR works closely with the Veterans Affairs and the Department of Defense (DOD) research arms to solve military medical problems. The most common reasons for American soldiers to be medically evacuated from combat theatre today are not injuries from jumping from planes or taking enemy fire. They’re musculoskeletal injuries, but largely from training and carrying around a rucksack.
In June, Pitt will host a national conference on human performance optimization, organized by collaborators across the health sciences. In September, the DOD will join in on a two-day meeting with Dr. Poropatich and others at Pitt to discuss how academia can fill in gaps in the DOD’s own efforts. “To open their eyes to areas of human performance optimization that they’re not currently thinking about,” says Dr. Poropatich.
But improving performance isn’t just in the interest of service members, of course. “There are lots of people at Pitt” chasing down this goal, says Dr. Cooper, “whether it’s post-transplant, or post–spinal cord injury, or amputation, or our own Division I athletes, or special operations forces.” Dr. Cooper has garnered numerous awards for his contributions to assistive technology, a field that enhances daily living for persons with disabilities. He’s also an elite athlete, medaling in the Paralympics and National Veterans Wheelchair Games year after year. (He’s racked up 150 Wheelchair Games medals.)
Dr. Fu, world renowned for innovating and evaluating surgical fixes for injured athletes’ knees, has made Pitt a powerhouse in probing the biomechanics of sports injuries. He helped conceptualize the UPMC Rooney Sports Complex, which opened in 2000 on the South Side. (It’s where the Steelers and Panthers do indoor training.) The sports medicine center there was just named for Dr. Fu. And in 2015, the UPMC Lemieux Sports Complex, a comprehensive outpatient sports medicine facility and training site for the Pittsburgh Penguins, opened in Cranberry. (“Nobody else has two sports centers,” Dr. Fu says.)
Dr. Fu oversees one of the largest, most comprehensive sports medicine clinical and research operations in the world. And he has worked to bring human performance optimization from the realm of the hocus-pocus to solid, replicable, peer-reviewed science.
In 1990, with an investment of $5,000 and half a classroom in Trees Hall, Dr. Fu founded the Neuromuscular Research Laboratory (NMRL). Slowly and steadily, NMRL grew, encompassing studies of a variety of athletes, as well as military service members. Three years ago, an entirely new facility dubbed the Neuromuscular Research Laboratory/Warrior Human Performance Research Center (NMRL/WHPRC) opened on Pittsburgh’s South Side.
Today, in the 11,600-square-foot space where lab meets gym, NMRL researchers study just about every physiological aspect of the human form in medias res: proprioception, postural stability, strength, range of motion, flexibility, bone and mineral density, you name it. There’s an on-site biochemistry lab, a suite of motion-capture cameras, a transcranial magnetic stimulator. (TMS is a noninvasive way of stimulating specific areas of the brain.) It even has a swimming flume and an underwater treadmill.
Dr. Nindl came on board as director when the new Neuromuscular Research Laboratory/Warrior Human Performance Research Center (NMRL/WHPRC) opened on Pittsburgh’s South Side. He calls it his dream job, and this physiology PhD does seem every bit the part. He’s a military guy—a reservist who served for 20 years as an Army Medical Department government scientist, primarily studying biomarkers for fitness and health outcomes.
In the past five years, exercise physiology research is complementing our expanding knowledge of health at the micro level, from head to toe. Human performance optimization is now more of a hybrid of both basic and applied science, Dr. Nindl says.
Dr. Nindl is a coprincipal investigator on a three-year study funded by the U.K. Ministry of Defence. SPARTA, which stands for Soldier Performance and Readiness as Tactical Athletes, will evaluate the efficacy of a variety of physical training regimens in preparing women for what’s required in close ground combat. “I’ve done a lot of work looking at both men and women and how they adapt,” says Dr. Nindl, “and I know that with optimal proper training you can significantly [close physical performance] gaps.”
Per Ms. Vitone, with all its research and clinical capabilities, Pitt, it seems, is uniquely positioned to anchor Pittsburgh as a Human Performance City.
“Zero Lift” Patient Transfer and Repositioning System
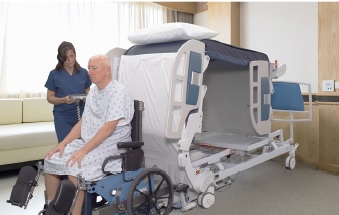
A new bed transfer device makes life easier — and safer — for patients and caregivers. NextHealth, Inc., in partnership with McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, and team members from University of Pittsburgh’s Human Engineering Research Laboratory (HERL), developed the AgileLife Transfer & Mobility System™ (“TMS”), the only “Zero Lift” transfer and repositioning system that affects better outcomes for immobile individuals, their families, caregivers and their providers through the continuum of care.
“What we are about is providing people devices that allow them to be as independent as possible, and also helping caregivers,” Dr. Cooper, FISA & Paralyzed Veterans of America (PVA) Professor and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine & Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh, and HERL Founding Director, said to M. Thomas of the Pittsburgh Post-Gazette. HERL has been involved in all aspects of the project, including development, writing software, fabrication and user trials.
Whether manually transferring or repositioning immobile individuals, or using mechanical devices, caregivers are at risk for musculoskeletal injuries. Sometimes these injuries go undetected over time and the compound effect of repeated manual strain can be debilitating. Absenteeism, retraining, compensation claims, permanent disability and early retirement, are some of the avoidable outcomes that generate unnecessary expense. Costs associated with patient transfer incidents are estimated to be $20 billion annually. This healthcare crisis has led to “Zero Lift” legislation being passed in many states. In contrast over a 12-month period, 10,000 transfers and over 20,000 in-bed repositions were completed and verified using the AgileLife TMS™. “Zero” injuries were reported.
The first unit in Pittsburgh was placed in the home of a patient at the VA H.J. Heinz campus in O’Hara Township. He had been in the facility for two years because his wife couldn’t lift him. After the AgileLife device was installed she could take him home, said Dr. Cooper.
The TMS also includes therapeutic mattress and seating options, and a tilt-in-space wheel chair that all combine to address individual positioning and skin integrity needs.
The enhanced R2.0 version, developed in partnership with HERL, has already been installed in assisted living and long-term care facilities and private homes. The Next Health-HERL team is working on the AgileLife R3.0 version which will accommodate a power wheelchair. At the earliest it would be launched at the end of this year.
The device may be rented, leased with the option to purchase or purchased outright. The cost, $44,800, is “not covered by insurance at this point,” said Raymond Curatolo, President of NextHealth, “but we are having conversations with insurers.”
Illustration: NextHealth, Inc.
Dr. Julie Phillippi Promotes STEM Excellence to Fox Chapel Area Students

As pioneers in the field of regenerative medicine, McGowan Institute for Regenerative Medicine faculty and staff are both obligated and delighted to contribute to the growth of the field by participating in the career development of young scientists. McGowan Institute for Regenerative Medicine faculty member Julie Phillippi, PhD, Assistant Professor in the Department of Cardiothoracic Surgery, School of Medicine, with a secondary appointment in the Department of Bioengineering, Swanson School of Engineering, recently participated in a panel discussion for sophomore and junior girls from the Fox Chapel Area High School sponsored by the American Association of University Women (AAUW). Students were encouraged to interact with Dr. Phillippi and other panel experts in an effort to expand their horizons while considering career options.
For their academic achievements in STEM fields, these young female students were honored by the AAUW. The girls were lauded during the ceremony at Fox Chapel Presbyterian Church by AAUW members from its Fox Chapel branch.
AAUW has a long history of opening doors for women and girls in science, technology, engineering, and mathematics (STEM), from the classroom to Capitol Hill. The STEM fields are rapidly becoming the most in-demand and lucrative in the world. Despite this demand, at almost every step of the STEM education path women and girls walk away. By middle school many girls are ambivalent toward these fields, and by the end of high school fewer girls than boys plan to pursue STEM studies in college.
Illustration: Dr. Julie Phillippi (far left) and fellow panelists.
Dr. Antonio D’Amore Weighs In on First 3D Printed Heart

In a major medical breakthrough, Tel Aviv University (TAU) researchers have ‘printed’ the world’s first 3D vascularized engineered heart using a patient’s own cells and biological materials. The engineered heart completely matches the immunological, cellular, biochemical and anatomical properties of the patient.
“This is the first time anyone anywhere has successfully engineered and printed an entire heart replete with cells, blood vessels, ventricles and chambers,” says Professor Tal Dvir of TAU’s School of Molecular Cell Biology and Biotechnology, Department of Materials Science and Engineering, Center for Nanoscience and Nanotechnology and Sagol Center for Regenerative Biotechnology, who led the study published in Advanced Science.
In response to this news, Pittsburgh CBS affiliate KDKA Radio reached out to McGowan Institute for Regenerative Medicine affiliated faculty member Antonio D’Amore, PhD, Research Assistant Professor in the Departments of Surgery and Bioengineering at the University of Pittsburgh, for his opinion of this groundbreaking innovation. He said this story of a 3D printed heart, from work started in 2006, is incremental and a proof-of-concept, but it affords great possibilities in the future. Dr. D’Amore explained the extreme difficulty associated with this accomplishment as he compared it to his own work creating heart valves through a process called electrospinning, a tissue engineering technique to create scaffolding to then create body organs.
Both co-hosts of the program, Marty Griffin and Wendy Bell, were amazed at the future treatments described by Dr. D’Amore and their potential availability to next generation patients.
Abstract (3D printing of personalized thick and perfusable cardiac patches and hearts. Nadav Noor, Assaf Shapira, Reuven Edri, Idan Gal, Lior Wertheim, Tal Dvir. Advanced Science, 2019; 1900344.)
AWARDS AND RECOGNITION
UPMC Insurance Services Division Names Joon S. Lee, MD, Chief Medical Officer

Joon S. Lee, MD, has been appointed Chief Medical Officer of the UPMC Insurance Services Division including its flagship the UPMC Health Plan. In this prominent role, Dr. Lee will provide medical leadership for the clinical team serving the 3.5 million members of the UPMC insurance and health management companies. He will direct clinical innovation efforts to improve health outcomes and will further advance UPMC’s goals as an integrated delivery and financing system. In addition to his role as Chief Medical Officer, he will continue his faculty appointment in the Department of Internal Medicine, University of Pittsburgh. Dr. Lee will report to Diane Holder, Executive Vice President of UPMC, President of Insurance Services and President and CEO UPMC Health Plan.
Dr. Lee is currently Division Chief of Cardiology at the University of Pittsburgh School of Medicine and Executive Director of UPMC’s Heart and Vascular Institute (HVI), one of the largest cardiovascular care delivery organizations in the country. He is recognized as an outstanding clinician, researcher, teacher, and innovator. An interventional cardiologist by training, Dr. Lee’s research and clinical interests include the role of stem cells in the treatment of coronary artery disease and catheter-based treatment of valvular disease. He has been instrumental in advancing innovation in clinical care at UPMC and recognized as an outstanding leader who is constantly pushing the envelope for new therapies to support recovery from cardiac related problems and prevention strategies to promote health and wellness. Dr. Lee is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Dr. Lee received his bachelor’s degree from Dartmouth College and his medical degree from Duke University’s School of Medicine. He completed a medical internship and residency, as well as two cardiology fellowships, at Massachusetts General Hospital in Boston. He joined the University of Pittsburgh faculty in 1996. Dr. Lee is certified in cardiovascular disease and interventional cardiology by the American Board of Internal Medicine. He is a member of the American College of Physicians and a fellow in the American College of Cardiology.
“I am very excited that Dr. Lee is joining UPMC Insurance Services Division in this major leadership role,” said Ms. Holder. “As a senior thought leader with extensive clinical and administrative experience, Dr. Lee is well positioned to support the transition to value-based care delivery models, lead translational research efforts, and enhance clinical care and innovative prevention and support programs for our members. As a practicing physician, Dr. Lee has vast experience in health care integration and offers a strong patient/consumer-focused approach.”
Robertson/Debski/Vorp Lab Students Participate in Three Minute Thesis Competitions

The Three Minute Thesis (3MT®) Competition, developed by The University of Queensland, helps higher degree students hone their research communication skills by challenging them to effectively explain their research in three minutes to a non-specialist audience. The University of Pittsburgh Swanson School of Engineering held an inaugural school-wide pre-qualifying event where the Department of Bioengineering swept all three awards.
Piyusha Gade (pictured top), a graduate student researcher in the lab of McGowan Institute for Regenerative Medicine affiliated faculty member Anne Robertson, PhD, Professor of Mechanical Engineering and Materials Science, captured first place in both the pre-qualifying event and the university-wide event. Her work involves rationally designing in situ engineered vascular grafts in young and aged hosts.
“I think effective science communication is extremely vital,” said Ms. Gade. “Especially today, it is not enough to just do good scientific work; you also need to communicate its relevance and impact to really make a change. Being a part of this competition helped me to take a step back and look at the big picture to understand where my work fits into it. I loved trying to figure out how to boil down five years-worth of research into three minutes!”
As the first-place winner, Ms. Gade will go on to compete in the 2019 Trans-Tasman 3MT Competition.
Gerald Ferrer (pictured center), a graduate student in the Orthopaedic Robotics Lab helmed by Bioengineering Professor and McGowan Institute affiliated faculty member Richard Debski, PhD, tied for second place in the pre-qualifying event with Aneesh Ramaswamy (pictured bottom), a graduate student in the Vascular Bioengineering Lab of McGowan Institute affiliated faculty member David Vorp, PhD, Associate Dean for Research and John A. Swanson Professor of Bioengineering.
Mr. Ferrer went on to win two prizes in the university-wide event: the runner-up prize and the People’s Choice award. His current research is focused on quantifying location specific mechanical properties in tendons using different ultrasound techniques and understanding key biomechanical factors that influence rotator cuff tear propagation through computational models.
“I enjoyed the challenge of communicating the significance and impact of our research in a way that everyone – regardless of their background – could understand and relate to, all in under three minutes,” Mr. Ferrer said. “Being able to communicate with a diverse audience is important because it allows us to bridge the gap between scientists and nonscientists thus increasing awareness of the societal impact of your research.”
3MT is designed to encourage students to communicate the importance of their research to the broader community. Since its launch in 2008, the 3MT competition has expanded to 67 countries, and events are currently held at more than 600 universities worldwide.
“The Three Minute Thesis competition provided an excellent opportunity for our graduate students to reflect on their work and strengthen their ability to clearly and effectively communicate their research,” said Mary Besterfield-Sacre, Nickolas A. Dececco Professor of Industrial Engineering and Associate Dean for Academic Affairs. “These professional development activities help inspire our students to pursue academic excellence, and I look forward to holding this event again next year.”
Congratulations, all!
Illustration: Gade (Research Gate); Ferrer (Debski Lab); Ramaswamy (sciVelo).
Krzysztof Matyjaszewski, PhD, Has Been Elected to the NAS

Carnegie Mellon University’s Krzysztof Matyjaszewski, PhD, has been elected to the National Academy of Sciences (NAS) in recognition of his distinguished and continuing achievements in original research. Dr. Matyjaszewski is an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
NAS membership is a widely accepted mark of excellence in science and is considered one of the highest honors that a scientist can receive.
Dr. Matyjaszewski, the J.C. Warner University Professor of the Natural Sciences in the Mellon College of Science’s Department of Chemistry, is world renowned for his discovery of atom transfer radical polymerization (ATRP), one of the most effective and widely used methods of controlled radical polymerization. ATRP has allowed for the creation of a wide range of materials with highly specific, tailored functionalities, including “smart” materials.
Dr. Matyjaszewski’s work has been licensed to companies worldwide and he has been cited in the scientific literature more than 100,000 times, making him one of the most cited chemists in the world. He was elected as a member of the National Academy of Engineering in 2006, a fellow of the American Chemical Society in 2010 and a fellow of the National Academy of Inventors in 2014. He is a member of Australian, Polish and Russian Academies of Sciences. He received the 2017 Benjamin Franklin Medal in Chemistry, the 2013 AkzoNobel North American Science Award, the 2011 Wolf Prize in Chemistry and the 2009 Presidential Green Chemistry Challenge Award, among many other awards.
Dr. Matyjaszewski is among 100 new members and 25 foreign associates to be elected to the academy in 2019.
NAS is a private, nonprofit institution that was established under a congressional charter signed by President Abraham Lincoln in 1863. It recognizes achievement in science by election to membership, and — with the National Academy of Engineering and the National Academy of Medicine — provides science, engineering and health policy advice to the federal government and other organizations.
Congratulations, Dr. Matyjaszewski!
Dr. Abhinav Humar Selected an Essential Pittsburgher

As Next Pittsburgh approached its fifth anniversary covering the people and places moving Pittsburgh forward, this milestone got them thinking: What will the next five years look like? Who are the people who will be breaking new ground, driving positive progress and innovating to build a better and brighter Pittsburgh for all of us?
Next Pittsburgh turned to its very committed audience of readers and asked: Who will lead change and keep improving this city in the next five years? Who are 25 essential people Pittsburgh cannot live without?
One of the 25 people selected this year includes McGowan Institute for Regenerative Medicine affiliated faculty member Abhinav Humar, MD, Chief, Division of Abdominal Transplantation Surgery, UPMC; Clinical Director, Thomas E. Starzl Transplantation Institute; Thomas E. Starzl Professor in Transplantation Surgery, and here’s why:
“If Pittsburgh is known for something, it’s known for transplant,” wrote one nominator about the history that has led to Dr. Humar’s work. “A pioneer in the development and refinement of new transplant procedures, Dr. Abhi Humar leads a team committed to reducing waiting list deaths through robust living-donor kidney and living-donor liver transplant programs, as well as other innovative methods to expand the donor pool. His team also partners with the University of Pittsburgh to advance basic science and clinically applied research, as well as to support the teaching and training of transplant specialists worldwide. Dr. Humar is respected by his peers and beloved by his patients. As the leader of our transplant program for more than a decade, he continues to push the limits of transplantation the same way Dr. Starzl did.”
Congratulations, Dr. Humar!
McGowan Institute for Regenerative Medicine 2019 Best Doctors

The McGowan Institute for Regenerative Medicine applauds its affiliated faculty members who were recently recognized by Pittsburgh Magazine. This year’s list was excerpted from The Best Doctors in America 2019-2020 database, which includes close to 40,000 U.S. doctors in more than 450 medical specialty/subspecialty combinations. The Best Doctors in America database is compiled and maintained by Best Doctors, Inc.
This year 33 McGowan Institute affiliated faculty were recognized in the May issue of the magazine with 4 colleagues* receiving dual specialty acknowledgment. Congratulations are extended to:
Anesthesiology: Erin Sullivan, MD
Cardiovascular Disease: Dennis McNamara, MD, John Pacella, MD
Critical Care Medicine: Derek Angus, MD, MPH, John Kellum, MD, FACP
Neurological Surgery: David Okonkwo, MD, PhD, Mark Richardson, MD, PhD, Elizabeth Tyler-Kabara, MD, PhD*
Neurology: Lawrence Wechsler, MD
Obstetrics and Gynecology: Stephen Emery, MD, Pamela Moalli, MD, PhD*
Ophthalmology: Ian Conner, MD, PhD
Orthopedic Surgery: Freddie Fu, MD, MaCalus Hogan, MD, Patrick McMahon, MD, Kurt Weiss, MD
Otolaryngology: Carl Snyderman, MD, MBA*
Pathology: Anthony Demetris, MD
Pediatric Cardiology: Jacqueline Kreutzer, MD
Pediatric Neurological Surgery: Elizabeth Tyler-Kabara, MD, PhD
Pediatric Surgery: George Gittes, MD, George Mazariegos, MD
Physical Medicine and Rehabilitation: Michael Boninger, MD
Plastic Surgery: Howard Edington, MD,* Michael Gimbel, MD, Ernest Manders, MD, Peter Rubin, MD, Kenneth Shestak, MD, Mario Solari, MD
Surgery: Timothy Billiar, MD, Abhinav Humar, MD, Carl Snyderman, MD, MBA
Surgical Oncology: Howard Edington, MD
Thoracic Surgery: Jonathan D’Cunha, MD, Thomas Gleason, MD, James Luketich, MD
Urology: Pamela Moalli, MD, PhD
Congratulations, all!
Tuan Lab Student Awarded 2019 NSF Graduate Research Fellowship

The National Science Foundation (NSF) Graduate Research Fellowship Program is designed to ensure the vitality and diversity of the scientific and engineering workforce in the United States. The program recognizes and supports outstanding students in science, technology, engineering and mathematics disciplines who are pursuing research-based master’s and doctoral degrees.
One of the awardees is Kalon Overholt, a bioengineering undergraduate student, who has worked under the mentorship of McGowan Institute for Regenerative Medicine affiliated faculty member Rocky Tuan, PhD, in the Center for Cellular and Molecular Engineering (CCME) for the past three years. Currently Dr. Tuan serves as the Vice-Chancellor and President of The Chinese University of Hong Kong.
Mr. Overholt’s research is focused on developing a device to study how biochemical crosstalk between bone and cartilage may contribute to the mechanism of osteoarthritis. He plans to pursue a graduate degree in biological engineering at the Massachusetts Institute of Technology starting in the Fall 2019.
The support accorded to NSF Graduate Research Fellows is intended to nurture awardees’ ambition to become lifelong leaders who contribute significantly to both scientific innovation and teaching. Fellows receive an annual stipend of $34,000 for three years, as well as a $12,000 cost of education allowance for tuition and fees.
Congratulations, Mr. Overholt!
Illustration: The University of Pittsburgh Biomedical Engineering Society.
Important Upcoming Dates
The Pennsylvania Pediatric Medical Device Consortium is accepting white paper applications for sponsored projects with up to 5 awards available for up to $50,000. Applications open up on Monday, June 3, 2019 and are due Friday, June 28, 2019.
Click Here for more information
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#197 –– Dr. Kris Dahl discusses her research using rheological, biophysical and optical techniques to understand the structure and organization of the cell nucleus.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Kokkinos V, Sisterson ND, Wozny TA, Richardson RM
Title: Association of Closed-Loop Brain Stimulation Neurophysiological Features With Seizure Control Among Patients With Focal Epilepsy
Summary: IMPORTANCE: A bidirectional brain-computer interface that performs neurostimulation has been shown to improve seizure control in patients with refractory epilepsy, but the therapeutic mechanism is unknown.
OBJECTIVE: To investigate whether electrographic effects of responsive neurostimulation (RNS), identified in electrocorticographic (ECOG) recordings from the device, are associated with patient outcomes.
DESIGN, SETTING, AND PARTICIPANTS: Retrospective review of ECOG recordings and accompanying clinical meta-data from 11 consecutive patients with focal epilepsy who were implanted with a neurostimulation system between January 28, 2015, and June 6, 2017, with 22 to 112 weeks of follow-up. Recorded ECOG data were obtained from the manufacturer; additional system-generated meta-data, including recording and detection settings, were collected directly from the manufacturer’s management system using an in-house, custom-built platform. Electrographic seizure patterns were identified in RNS recordings and evaluated in the time-frequency domain, which was locked to the onset of the seizure pattern.
MAIN OUTCOMES AND MEASURES: Patterns of electrophysiological modulation were identified and then classified according to their latency of onset in relation to triggered stimulation events. Seizure control after RNS implantation was assessed by 3 main variables: mean frequency of seizure occurrence, estimated mean severity of seizures, and mean duration of seizures. Overall seizure outcomes were evaluated by the extended Personal Impact of Epilepsy Scale questionnaires, a patient-reported outcome measure of 3 domains (seizure characteristics, medication adverse effects, and quality of life), with a range of possible scores from 0 to 300 in which lower scores indicate worse status, and the Engel scale, which comprises 4 classes (I-IV) in which lower numbers indicate greater improvement.
RESULTS: Electrocorticographic data from 11 patients (8 female; mean [range] age, 35 [19-65] years; mean [range] duration of epilepsy, 19 [5-37] years) were analyzed. Two main categories of electrophysiological signatures of stimulation-induced modulation of the seizure network were discovered: direct and indirect effects. Direct effects included ictal inhibition and early frequency modulation but were not associated with improved clinical outcomes (odds ratio [OR], 0.67; 95% CI, 0.06-7.35; P > .99). Only indirect effects-those occurring remote from triggered stimulation-were associated with improved clinical outcomes (OR, infinity; 95% CI, -infinity to infinity; P = .02). These indirect effects included spontaneous ictal inhibition, frequency modulation, fragmentation, and ictal duration modulation.
CONCLUSIONS AND RELEVANCE: These findings suggest that RNS effectiveness may be explained by long-term, stimulation-induced modulation of seizure network activity rather than by direct effects on each detected seizure.
Source: JAMA Neurology. 2019 Apr 15. doi: 10.1001/jamaneurol.2019.0658. [Epub ahead of print]
GRANT OF THE MONTH
PI: Pamela Moalli
Co-PI: Steven Abramowitch
Title: Overcoming Complications of Polypropylene Prolapse Meshes: Development of Novel Elastomeric Auxetic Devices
Description: Pelvic organ prolapse (POP) is a common, costly condition in women with a lifetime risk of surgical repair of 12.6%. Of those undergoing a native tissue repair, 40% will fail by 2 years prompting surgeons to turn to biomaterials, most commonly polypropylene mesh. Unfortunately, POP meshes are abdominal hernia meshes that have been remarketed under 510K applications for POP repair and, thus, were never designed specifically for the vagina. Our studies show that implantation with polypropylene mesh leads to degeneration, atrophy and loss of function of the vagina. The high material stiffness of polypropylene dictates that meshes manufactured from this polymer are knitted, leading to a device that undergoes pore collapse, wrinkling, and permanent deformation — all contributing to increased mesh burden, a heightened foreign body response, and poor outcomes. We hypothesize that a mesh generated from an elastomeric polymer with a material stiffness on the same order of magnitude as that of the vagina, a geometry that favors stable pores, and minimal wrinkling with tensioning will be associated with a more favorable host response than current polypropylene prolapse meshes. Here we are proposing to develop and evaluate a mesh synthesized from polycarbonate-urethane (PCU), an elastomer with an inherent stiffness similar to that of vagina but that is also sufficiently tough to meet physiologic loading demands. The mesh is designed with auxetic pores; meaning they expand instead of contract with loading. In addition, the mesh can be 3D printed, permitting us to fine tune the in-plane geometry and thickness, and allow for non-rotational junctions, thereby reducing wrinkling and permanent deformation. Specifically, our goal in this application is to delineate the impact of our choice for the stiffness of PCU on the host response to the mesh because this choice will impact the amount of material necessary to achieve structural support and strength equivalent to that of polypropylene mesh. The amount of material contributes directly to the magnitude of the host response, but can also obviate or enhance mechanical behaviors, e.g. wrinkling, in the device that feedback into the host response. Thus, in moving this device forward for eventual use in humans, we will study how our design choices independently impact the host response to the material and the mechanics of the mesh by: (Aim 1) implanting small units of the mesh with varied material stiffness, fiber width and mesh thickness on the vagina without tensioning and loading, and (Aim 2) utilizing computational modeling and ex vivo tests to determine the impact of the same design choices on the mechanical behavior of the full length mesh with loading. In Aim 3, we will study how choices that balance the host response to the material with those resulting from the mechanics of the mesh collectively contribute to the overall host response to a mesh that is implanted on tension by sacrocolpopexy in a validated animal model as compared to conventional polypropylene mesh. In this way, the device developed in this proposal has high potential for markedly improving outcomes in POP surgical repair.
Source: Eunice Kennedy Shriver National Institute of Child Health & Human Development
Term: April 17, 2019 – March 31, 2024
Amount: $542,271 (2019)
