March 2019 | VOL. 18, NO. 03| www.McGowan.pitt.edu
McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat

The McGowan Institute for Regenerative Medicine held its 2019 Scientific Retreat March 11-12, 2019. The focus was on peer-to-peer networking, and the retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful discussions and identification of opportunities for partnerships. This year’s program committee (listed below) designed an exciting program:
- Julie Phillippi, PhD (Chair) – University of Pittsburgh
- Bryan Brown, PhD – University of Pittsburgh
- Patrick Cantini – University of Pittsburgh
- Garrett Coyan, MD (trainee member) – University of Pittsburgh
- Andy Duncan, PhD – University of Pittsburgh
- Adam Feinberg, PhD – Carnegie Mellon University
- George Gittes, MD – Children’s Hospital of UPMC
- Jelena Janjic, PhD – Duquesne University
- Robert Kormos, MD – University of Pittsburgh
Program Highlights
The Director’s Welcome and State of the Institute Address was presented by McGowan Institute Director William Wagner, PhD, Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. Dr. Wagner also introduced the Distinguished Lecturer, Jennifer Elisseeff, PhD, Professor of Ophthalmology, Director of the Translational Tissue Engineering Center, Johns Hopkins University. Dr. Elisseeff’s presentation was entitled, “Lessons in Regenerative Medicine Translation: Are We Designing for the Right Therapeutic Target?”
A first-time-ever, open-to-the-public program was also held. “Stem Cell Therapies: Hope vs Hype” focused on understanding both the potential and the limitations of stem cells. Bill Flanagan, chief corporate relations officer, Allegheny Conference on Community Development, served as the panel moderator. Panelists included:
- Lawrence Wechsler, MD, Chair, Department of Neurology, University of Pittsburgh School of Medicine
- Carl Kurlander, documentarian and filmmaker, Visiting Distinguished Senior Lecturer, University of Pittsburgh
- Peter Rubin, MD, chair, Department of Plastic Surgery, University of Pittsburgh
A video of this discussion can be viewed here.
The Retreat included numerous general sessions throughout the 2-day event with a focus on these research and/or strategic planning areas:
- Pulmonary Bioengineering/Biohybrid Organs
- Importance of (Re)Vascularization in Tissue Regeneration
- Computational Modeling of Injury, Infection, Inflammation, and Healing
- Regenerative Rehabilitation
- Pediatric Device Initiative
- Drug Delivery Strategies in the Extracellular and Intracellular Space
- Tumor Immunotherapy: Novel Research Foci and Improved Clinical Targets
- Emerging Military Threats, Combat Casualty Gaps and Opportunities for Innovation
- Liver Regeneration
- Image-Guided Drug Delivery in Regenerative Medicine
- Let’s Talk Science: Communicating Your Research to Different Audiences
The Retreat program also included 88 presentations addressing a great cross-section of scientific topics given by McGowan Institute affiliated faculty and 57 invited researchers from other institutions and agencies who are not formally affiliated with the McGowan Institute (in alphabetical order):
- Peter Alexander, PhD, Assistant Professor, Department of Orthopaedic Surgery, University of Pittsburgh: “Development of Human MSC-Based Organotypic Cultures for Toxicity Testing”
- Carl Anderson, PhD, Division Head, Pharmaceutical, Administrative & Social Sciences, Duquesne University School of Pharmacy: “Process Analytical Technology (PAT) Technology and Applications”
- Ali Aral, MD, Postdoctoral Scholar, Department of Surgery, University of Pittsburgh Poster 40: “Elucidation and Integration of Tissue-Specific, Protein-Level Inflammatory Networks following Vascularized Composite Allotransplantation”
- David Bartlett, MD, Bernard Fisher Professor of Surgery, University of Pittsburgh School of Medicine: “Oncolytic Vaccinia Virus Delivering Cytokines – Trials and Tribulations”
- Michael Behrens, BS, PhD Student, Department of Bioengineering, University of Pittsburgh Poster 4: “Biomagnetic Genetically Programmed Multicellular Microrobots”
- Andrew Bradshaw, BS, PhD Student, Department of Pathology, University of Pittsburgh: “The Therapeutically Induced Matrisome (Tim) Protects and Promotes Metastatic Disease”
- Donna Brezinski, MD, CEO and Founder, Little Sparrows Technologies, Inc.: “Bili-Hut™: Merging Human Centered Design, Usability, Biomedical Engineering and Health Economics to Democratize Newborn Jaundice Treatment”
- Tullia Bruno, PhD, Research Assistant Professor, Department of Immunology, University of Pittsburgh: “Tumor Infiltrating B Cells: An Immunotherapy Target on the Rise”
- Maysam Chamanzar, PhD, Assistant Professor, Department of Electrical and Computer Engineering, Carnegie Mellon University: “Parylene Photonics: A New Platform for Flexible Optoelectronic Sensors”
- Ryan Champagne, Grants Development Coordinator, Office of Research, University of Pittsburgh: “Finding Money”
- Stephen Chan, MD, PhD, FAHA, Director, Center for Pulmonary Vascular Biology and Medicine; Associate Professor of Medicine, Pittsburgh Heart, Lung, Blood, and Vascular Medicine Institute, Division of Cardiology, Department of Medicine, UPMC and University of Pittsburgh School of Medicine: “Iron-Sulfur Biogenesis Controls Endothelial Dysfunction in Pulmonary Hypertension”
- David Chi, MD, Associate Professor, Department of Otolaryngology, University of Pittsburgh; Clinical Director, Division of Pediatric Otolaryngology, UPMC Children’s Hospital of Pittsburgh: “Biodegradable Magnesium Alloy-Based Tracheal Stent”
- Vaughn Cooper, PhD, Professor, Department of Microbiology and Molecular Genetics, University of Pittsburgh: “Being Social: Dos and Don’ts of Talking Science on Social Media”
- Leonardo D’Aiuto, PhD, Research Instructor, Department of Psychiatry, University of Pittsburgh: “iPSC-Based Three-Dimensional Neuronal Platforms for Modeling CNS Infections and Drug Screening”
- Greg Delgoffe, PhD, Assistant Professor, Department of Immunology, University of Pittsburgh: “Engineering Metabolic Sufficiency in Adoptive Cell Therapies for Cancer”
- Cameron Dezfullian, MD, Assistant Professor, Adult and Pediatric Critical Care Medicine and Clinical and Translational Science; Scientist, Safar Center for Resuscitation Research; Faculty, Vascular Medicine Institute, University of Pittsburgh School of Medicine: “ThreadRiteIV Catheter”
- Rajeev Dhupar, MD, MBA, Assistant Professor of Cardiothoracic Surgery; Chief, Thoracic Surgery, VAMC of Pittsburgh; Department of Cardiothoracic Surgery, University of Pittsburgh: “Novel Immune Approaches to the Pleural Space”
- James K. Drennen, PhD, Associate Dean for Research and Graduate Programs, Duquesne University School of Pharmacy: “Quality by Design: Modern Strategies for Product Development”
- Chigozirim Ekeke, MD, Cardiothoracic Surgery Resident, UPMC: “Current Therapies for Malignant Pleural Effusion”
- Swaytha Ganesh, MD, Assistant Professor, Department of Medicine—Division of Gastroenterology, Hepatology, and Nutrition; Medical Director of Living Donor Program—UPMC Thomas E. Starzl Transplantation Institute, Center for Liver Diseases, University of Pittsburgh: “Liver Regeneration Making Living Donation Feasible”
- Jorge Genovese, MD, PhD, Vice President of Electrical Stimulation Regeneration Research, BioLeonhardt LTP; Cal-X Starts Business Accelerator; Leonhardt’s Launchpads Utah: “Bioelectric Medicine: A Regenerative Approach”
- Anne Germain, PhD, Professor of Psychiatry, Psychology, and Clinical and Translational Science (on leave), University of Pittsburgh; CEO, Noctem LLC: “Life Sciences Start-Ups: Lessons from the First Year”
- Miroslaw Janowski, MA, MD, PhD, Associate Professor, Department of Radiology, Johns Hopkins University: “Interventional PET for Precise and Quantitative Monitoring of the Delivery of Biologicals to the Brain”
- Jana Kainerstorfer, PhD, Assistant Professor, Department of Biomedical Engineering, Carnegie Mellon University: “Monitoring of Cerebral Perfusion Continuously and at the Bedside with Near-Infrared Light”
- Arthur Levine, MD, Senior Vice Chancellor for the Health Sciences, John and Gertrude Petersen Dean, University of Pittsburgh School of Medicine: “Institutional Environment and Support for New/Early Stage Investigators”
- Jingjing Li, MD, Research Fellow, Department of Plastic Surgery, University of Pittsburgh: “Local Delivery of Adipose-Derived Mesenchymal Stem Cells Promotes Immunomodulation and Allograft Survival in Vascularized Composite Allotransplantation (VCA)”
- Zhong Li, PhD, Postdoctoral Associate, Department of Orthopaedic Surgery, University of Pittsburgh: “Organ-on-a-Chip System for the Modeling of Synovial Joint Pathologies”
- Lu Liu, MS, PhD Candidate, Graduate School of Pharmaceutical Sciences, Chronic Pain Research Consortium, Duquesne University Poster 76: “Theranostic Analgesic Regenerative Gel-Emulsion Technology (T.A.R.G.E.T.) Platform for Local Analgesia and Promotion of Nerve Regeneration”
- Jason Lohmueller, PhD, Postdoctoral Associate, Department of Immunology, University of Pittsburgh: “Engineering Universal CAR and SynNotch Receptors for Programmable Antigen Targeting”
- Katherine Lorentz, MS, PhD Student, Department of Bioengineering, University of Pittsburgh Poster 100: “Cytokine Mimicking Microspheres for Use in Porous Scaffolds”
- Marcus Malek, MD, FAAP, Assistant Professor of Surgery; Director, Pediatric Surgical Oncology, Department of Surgery, University of Pittsburgh: “PET Probe for Radioguided Surgery”
- Hikaru Mamiya, BS, PhD Student, Department of Bioengineering, University of Pittsburgh: “Exposure of Muscle Stem Cells to a Stiff Microenvironment Drives an “Aged” Mitochondrial Phenotype”
- Devika S. Manickam, PhD, Assistant Professor of Pharmaceutical Sciences, Duquesne University: “Nanoparticle-mediated CNS Drug Delivery”
- Shibin Mathew, PhD, Postdoctoral Research Fellow, Department of Cancer Biology, Dana Farber Cancer Institute; Department of Genetics, Harvard Medical School: “Computing Stem Cell Fate Choice for Regenerative Medicine Applications”
- Michael McClincy, MD, Assistant Professor, Department of Orthopaedic Surgery, University of Pittsburgh: “interACTION: ACL”
- Christine McDonough, PT, PhD, Assistant Professor, Departments of Physical Therapy and Orthopaedic Surgery, University of Pittsburgh School of Medicine: “Evaluating Clinical Outcomes of Biological Treatments”
- Mara McFadden, MBA, VP of Product and Medical Devices, LifeX Ventures: “Regional Resources for Taking a Company from Concept to Start-Up”
- Charlie O’Hanlon, President and CEO, CelSense: “Development of Imaging Nanoparticles for Translational MR Imaging in Regenerative Medicine”
- Ryan Orizondo, PhD, Postdoctoral Associate, McGowan Institute, University of Pittsburgh: “Thirty-Day In Vivo Evaluation of a Wearable Pumping Artificial Lung”
- Burak Ozdoganlar, PhD, Professor, Department of Mechanical Engineering, Carnegie Mellon University: “Microneedles Arrays for Transdermal and Intradermal Deliver of Bioactive Molecules and their Application for Endogenous Sensing”
- Vaibhav P. Pai, PhD, Research Scientist II, Allen Discovery Center, Tufts University: “Exploiting Endogenous Bioelectric Signaling for Regenerative Medicine”
- Monica Pearl, MD, DABR, Associate Professor, Interventional Neuroradiology; Director, Interventional Neuroradiology Fellowship Program; Director of Faculty Experience, Department of Radiology, Johns Hopkins University: “Unmet Needs and Opportunities for Regenerative Medicine in Interventional Neuroradiology”
- Ngoc Pham, BS, PhD Student, Graduate School of Pharmaceutical Sciences, Duquesne University: “Kinetic Model Simulation and Antigenicity Analysis of a Miniaturized Fc-Binding Domain for Local Deposition of Antibodies”
- Joseph Pugar, CEO, Aruga Technologies LLC: “Life Sciences Start-Ups: Lessons from the First Year”
- Liz Reid, Health & Science Editor, 90.5 WESA FM: “Science in the News: What Makes Your Research Interesting to a Reporter”
- Nicholas L. Robbins, DO, Surgical Resident, AIRMED, 59th MDW, U.S. Air Force: “Prevention of Ischemia-Reperfusion Injury and Chronic Rejection in a Porcine Vascularized Composite Allotransplantation Model”
- Jacquelyn Russell, BS, PhD Student, Department of Pathology, University of Pittsburgh: “Lack of Beta-Catenin in Hepatocytes Impairs Proliferation and Promotes Liver Progenitor Cell-Mediated Repair in Response to Hepatic Injury”
- Margaux Salas, PhD, Senior Scientist, AIRMED, 59th MDW, U.S. Air Force: “Prolonged Field Care on the Multi-Domain Battlefield: Current Emphasis and Future Needs”
- Pablo G. Sanchez, MD, PhD, FACS, Assistant Professor of Cardiothoracic Surgery; Vice Chairman, Benign Lung Diseases; Surgical Director, Lung Transplantation and ECMO; Director, Lung Transplant Research; Director, Ex Vivo Lung Perfusion (EVLP) Program, Department of Cardiothoracic Surgery, University of Pittsburgh: “Clinical Aspects of Ex Vivo Lung Perfusion”
- Benjamin K. Schilling, MS, PhD Student, Department of Bioengineering, University of Pittsburgh Poster 106: “Adipose-Derived Stem Cells Partially Mitigate Muscle Atrophy after Peripheral Nerve Injury in the Rodent Model”
- Michael Sippel, MD, Surgical Resident, AIRMED, 59th MDW, U.S. Air Force: “The AIRMED Program: Regenerative, Resuscitative and Restorative Technologies in Combat Casualty Care”
- Brian Stancil, PhD, President, Lifeware Labs: “Continuous Monitoring and Treatment Opportunities, Applications and Challenges Using Biosensing Stickers”
- Antonio Torres, Entrepreneur in Residence, University of Pittsburgh: “Business Basics: Pitching your Invention to Potential Partners”
- Piotr Walczak, PhD, Associate Professor, Department of Radiology, Johns Hopkins University: “Image-Guided Intra-Arterial Delivery of Cell Therapy for Stroke”
- Mallory R. Wampler, MD, Postdoctoral Scholar, AIRMED/RESTOR, United States Army Institute of Surgical Research: “Theranostic Analgesic Regenerative Gel-Emulsion Technology (T.A.R.G.E.T.) Platform for Local Analgesia and Promotion of Nerve Regeneration”
- Jacqueline Wittmer, BS, PhD student, Biomedical Engineering, Carnegie Mellon University Poster 110: “Perfused 3D Printed Collagen Tubes Support Tissue Viability”
- Sai Yerneni, BS, PhD Student, Department of Biomedical Engineering, Carnegie Mellon University: “Decellularized Chick Chorioallantoic Membrane as an Inductive Biomaterial for Functional Blood and Lymph Vasculature”
Poster Session
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. McGowan Institute faculty member Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
Cell and Gene Therapy
First Place
Jingjing Li
Local Delivery of Adipose-Derived Mesenchymal Stem Cells Promotes Immunomodulation and Allograft Survival in Vascularized Composite Allotransplantation (VCA)
Mentor: Mario Solari, MD
Dept. of Plastic Surgery
University of Pittsburgh
Second Place
Daniel Zuppo
Foxm1 drives Cardiomyocyte Proliferation during Zebrafish Cardiac Regeneration
Mentor: Michael Tsang, PhD
Dept. of Developmental Biology
University of Pittsburgh
Third Place
Kyle Sylakowski
The Angiogenic Capability of Mesenchymal Stem Cells Coupled with Tenascin-C Under Hypoxic Conditions
Mentor: Alan Wells, MD, DMS
Dept. of Pathology
University of Pittsburgh
Computation and Modeling
First Place
Leonid Emerel
Pre-Dissection-Derived Geometric and Distensibility Indices Reveal Increased Peak Longitudinal Stress and Stiffness in Patients Sustaining Acute Type A Aortic Dissection: Implications for Predicting Dissection
Mentors: Thomas Gleason, MD, Julie Phillippi, PhD, Marie Billaud, PhD
Dept. of Cardiothoracic Surgery, McGowan Institute
University of Pittsburgh
Second Place
George Gabriel
Characterization of Neurodevelopmental Defects Associated with a Mouse Model of Hypoplastic Left Heart Syndrome
Mentor: Cecilia Lo, PhD
Dept. of Developmental Biology
University of Pittsburgh
Third Place
Ronald Fortunato
Failure Biomechanics of Arterial Tissue
Mentor: Spandan Maiti, PhD
Dept. of Mechanical Engineering & Materials Science
University of Pittsburgh
Medical Devices
First Place
Alexis Nolfi
Polyelectrolyte multilayer coating for delivery of IL-4 from contact lenses for dry eye disease
Mentor: Bryan Brown, PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Second Place
Edgar Aranda-Michel
Beating Heart Simulation of Left Ventricular Compression for Heart Failure
Mentor: Dennis Trumble, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Third Place
Catherine Go
A Novel Retrievable Rescue Stent as a Comprehensive Solution to Non-Compressible Traumatic Hemorrhage
Mentor: Bryan Tillman, MD, PhD
Dept. of Surgery, McGowan Institute
University of Pittsburgh
Tissue Engineering
First Place
Saigopalakrishna Yerneni
Transdermal Delivery of Extracellular Vesicles Using Dissolvable Microneedle Arrays to Control Inflammation
Mentor: Phil Campbell, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Second Place
Sahil Rastogi
Novel Three-Dimensional Fuzzy Graphene (3DFG)-Based Ultra Microelectrodes Array for Sub-Cellular Electrical Recordings
Mentor: Tzahi Cohen-Karni, PhD
Dept. of Biomedical Engineering
Carnegie Mellon University
Third Place
Michael Buckenmeyer
The Development of Ovarian Hydrogels as an Alternative Strategy for Fertility Preservation
Mentor: Bryan Brown PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
People’s Choice
Ngoc Pham
Kinetic Model Simulation and Antigenicity Analysis of a Miniaturized Fc-Binding Domain for Local Deposition of Antibodies
Mentor: Wilson Meng, PhD
Graduate School of Pharmaceutical Sciences
Duquesne University
Poster Awards Sponsored by sciVelo
First place: $200 (each category)
Second place: $125
Third place: $75
CATER Program
First Place
Alexis Nolfi
Polyelectrolyte multilayer coating for delivery of IL-4 from contact lenses for dry eye disease
Mentor: Bryan Brown PhD
Dept. of Bioengineering, McGowan Institute
University of Pittsburgh
Second Place
George Gabriel
Characterization of Neurodevelopmental Defects Associated with a Mouse Model of Hypoplastic Left Heart Syndrome
Mentor: Cecilia Lo, PhD
Dept. of Developmental Biology
University of Pittsburgh
Third Place
Andrew Bradshaw
The Therapeutically Induced Matrisome (Tim) Protects and Promotes Metastatic Disease
Mentor: Alan Wells, MD, DMS
Dept. of Pathology
University of Pittsburgh
Awards by CATER
First place: $200
Second place: $125
Third place: $75
A special thank you is extended to all who made this year’s Retreat a success!
See the full Retreat Program here.
Photos Credit: Aimee Colabine Beattie.
RESOURCES AT THE MCGOWAN INSTITUTE
April Histology Special
April Showers mean big savings at the McGowan Histology Core Lab.
Herovici stain is used to differentiate young and mature collagen as well as identify reticulum fibers within tissue. Herovici’s solution stains young collagen and reticulum blue and mature collagen red while providing a yellow cytoplasm counterstain. Nuclei are stained blue to black with Weigert’s Hematoxylin.
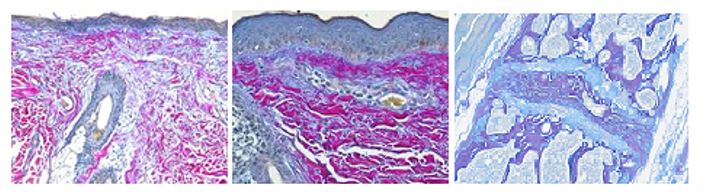
You’ll receive 25% off Herovici Staining for the entire month of April when you mention this ad.
Contact the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology work with the quickest turn-around time.
AWARDS AND RECOGNITION
2019 CSC Awards Winner: Dr. William Federspiel

The 23nd Annual Carnegie Science Awards celebration will be held on May 10, 2019, at Carnegie Science Center’s new PPG Science Pavilion during which time McGowan Institute for Regenerative Medicine faculty member William Federspiel, PhD will be presented with the Life Sciences Award for his tremendous work and its impact on the vitality in the region.
The Carnegie Science Center established the Carnegie Science Awards program in 1997 to recognize and promote outstanding science and technology achievements in western Pennsylvania.
The Carnegie Science Awards have honored the accomplishments of more than 600 individuals and organizations whose contributions in the fields of science, technology, and education have impacted our region’s industrial, academic, and environmental vitality.
The Life Sciences Award recognizes and honors scientific advances in new and innovative biomedical and life sciences endeavors that benefit the economy, health, or societal wellbeing of the region. Dr. Federspiel is a scientist and an innovator who has significantly advanced the state-of-the-art in several critical areas through the design and demonstration of innovative medical devices for critical care medicine. Dr. Federspiel’s research interests include the following areas:
- Design and development of novel artificial lung devices, including respiratory support catheters and paracorporeal assist lungs, for near-term clinical use in the treatment of respiratory failure in patients with acute, acute on chronic, or chronic lung insufficiencies.
- Design and development of membrane and particle-based blood purification devices for the selective or semi-selective and patterned removal of pathogenic antibodies, inflammatory mediators, and other blood borne solutes for near-term clinical use in critical care settings.
- Development of oxygen depletion devices for blood storage systems that will extend the shelf life of red cell units and deliver red cells of higher efficacy and lower toxicity for transfusion therapy.
- Advanced application of fluid mechanics and mass transport principles to model and optimize artificial lungs and other membrane-based medical devices where functional performance depends on underlying transport or separation principles that dictate the device characteristics.
- Development of mathematical and computer simulation models related to respiratory and cardiovascular fluid mechanics and mass transport.
Dr. Federspiel perhaps has the most “bench to bedside” experience of any scientist in the region, which has been gained from developing and commercializing the Hemolung RAS. This experience has given him a valuable perspective on the clinical translation of medical devices from the concept stage to clinical implementation, and he is effectively using it to bring new lifesaving technologies to the physicians and patients in need.
Congratulations, Dr. Federspiel!
Also, honorable mentions were awarded in 10 categories. Four of the categories were won by McGowan Institute affiliated faculty/students. They include:
- Advanced Manufacturing and Materials – Catalina Pineda-Molina, The Badylak Lab
- Life Sciences – Dietrich A. Stephan, PhD, LifeX
- Postsecondary Educator – Bryan Brown, PhD, Assistant Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh
- College/University Student – Alexis Nolfi, The Brown Lab
Congratulations, all!
SCIENTIFIC ADVANCES
Harnessing the Power of the Lymph Node

Do lymph nodes hold the key to regenerating failed organs?
Our body’s lymph nodes generally go unnoticed, unless they become swollen and painful in the fight against infection. They’re part of a body-wide system that quietly grows immune cells, circulates them to infection sites, and carries away waste.
Recently, however, the unique efficiency of lymph nodes in developing cells is putting them at the forefront of an exciting bioengineering effort that could someday lead to the replacement of organs lost to disease.
The Ultimate Bioreactor
Eric Lagasse, PharmD, PhD, Director of the Cancer Stem Cell Center at the University of Pittsburgh’s McGowan Institute for Regenerative Medicine, describes the lymph node as the “ultimate bioreactor” that can potentially outdo any piece of lab equipment when it comes to regenerating cells. “It has access to the blood system, the lymph system, and everything else needed to support growing cells,” he says.
More importantly, the lymph node plays an integral role in a little-understood mechanism that directs cell progenitors (stem cells and other building blocks) to become fully developed, specialized cell structures that can perform important body functions. Scientists like Dr. Lagasse, Associate Professor in the Department of Pathology, University of Pittsburgh, with a secondary appointment in Pitt’s Clinical and Translational Institute, are hoping that what the lymph node can do for immune cells, it can do for liver, kidney, and other cells.
For nearly a decade, Dr. Lagasse has been growing functional liver, thymus, pancreas, kidney and other tissues in animal lymph nodes. His cell research has passed from small to large animal experiments and is now awaiting approval for clinical trials at UPMC and other progressive medical centers around the country. But it’s the McGowan Institute’s kidney research that could yield the most meaningful results.
The Disease with No Cure
Few human diseases compare to kidney disease, with its relatively high rate of occurrence, a life-threatening end stage, and the complete lack of a cure. “In kids, cancer has an 80 percent cure rate. End stage kidney disease failure has zero,” says Carlton Bates, MD, chief of pediatric nephrology at UPMC Children’s Hospital of Pittsburgh. “Chronically damaged kidneys don’t get better. The treatment is dialysis, which can dramatically shorten a life. And kidney transplantation is fraught with a chronic shortage of donors. That’s why there’s so much interest in this research.”
Over the last several years, Dr. Lagasse, Dr. Bates, and others at UPMC and the University of Pittsburgh have been exploring the lymph node’s remarkable regenerative ability. In research first published in 2016, Dr. Lagasse demonstrated that deconstructed mouse kidney tissue, placed in a mouse lymph node, could develop into functional kidney structures that could filter blood and produce urine. The team is now exploring the process at the cellular level, using induced pluripotent stem cells in their efforts to identify the complete set of progenitor materials that would form an actual kidney. As research progresses from mice to larger animals, the data will provide a better picture of how this process might be successful using human lymph nodes.
A Kidney from a Lymph Node?
Could the day come when doctors are able to transform a lymph node into a fully integrated, fully functional kidney inside a patient’s body? “It’s a possibility,” says Dr. Bates. “But what’s more promising in the near term is the understanding this work gives us of the mechanisms of growing tissue inside the body instead of a petri dish. This process is more robust and more productive, and it lets us see the results in a living system. It could point us toward an even better way to someday replace an organ inside the body.”
Extracellular Matrix: Communication Matters

In the lab of McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor of surgery, University of Pittsburgh, and director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute, the major focus is the development of regenerative medicine strategies for tissue and organ replacement. The utilization of mammalian extracellular matrix (ECM), or its derivatives, as an inductive template for constructive remodeling of tissue is a common theme of most research activities.
“If you asked anybody 25 years ago about the function of the extracellular matrix, they would’ve said it was structural,” says Dr. Badylak. “Now it’s the opposite: it’s recognized as a reservoir of signaling molecules that serves as a sort of information highway between cells.”
By the mid-1980s, Dr. Badylak had begun to explore whether healthy matrices stripped of their cells could be used to stimulate tissue regeneration in animals. This stripping process, dubbed decellularization, involves treating tissues with a mix of chemicals, including detergents and enzymes, to remove cells while leaving the matrix intact.
Although ECMs vary from tissue to tissue, therapeutic materials can originate from different organs or even different species. For some tissues, it almost doesn’t matter what ECM is used, Dr. Badylak says; for others, “it definitely makes a difference.”
For instance, matrices from the pig small intestine, bladder or dermis can all repair human skeletal muscle. But for the esophagus, only an ECM from the same tissue will suffice. And, in the central nervous system (CNS), a foreign matrix works better than a nervous system one. Dr. Badylak’s team found that urinary bladder ECM stimulates neuronal stem cells to proliferate better than an ECM derived from the CNS.
In their project on CNS Tissue Engineering, the objective of the Badylak Lab’s present and future work is to develop an effective substrate for reconstruction of functional CNS tissue. We believe that the ECM provides such a substrate and can “jumpstart” regeneration of CNS tissue either by recruitment of endogenous stem cells to the site of injury or by placement of such stem cells within a scaffold that is placed at the injury site.
Jyoti Madhusoodanan reports in her Nature article on Dr. Badylak’s and others’ work and the ECM and how it governs a surprising number of cellular functions. New science techniques are revealing how cells and matrix communicate — and why this cross-talk matters.
Genomics Could Guide Pancreatic Cancer Treatment

Pancreatic cancer is a grim diagnosis, with a five-year survival rate of less than 9 percent. To improve those odds, researchers at UPMC and the University of Pittsburgh School of Medicine sought genetic signatures in the largest study of its kind that could be used to better match drugs to patients and for early detection.
The study, published in the journal Gastroenterology, involved sifting through the genomes of thousands of tumors, sampled from all over the world. In 17 percent of cases, there was a genetic flag that indicated the tumor should be susceptible to existing chemotherapy drugs. The researchers also found supporting evidence for heritable genes, including some in the BRCA family associated with breast cancer, that can predispose whole families toward pancreatic cancer.
“People have been looking for such markers for a long time, and our study shows that it’s possible to break pancreatic cancer patients into different treatment buckets,” said senior author and McGowan Institute for Regenerative Medicine affiliated faculty member Nathan Bahary, MD, PhD, oncologist at UPMC Hillman Cancer Center and associate professor of medicine at Pitt.
One reason why pancreatic cancer is so deadly is that the majority of patients often are identified late in their disease course and frequently present with inoperable tumors at the time of diagnosis. For some of these patients, it may be possible to shrink the tumor with existing chemotherapy drugs, but in a disease where 75 percent of patients die within a year of diagnosis, time is of the essence, and unfortunately, there’s no way to know in advance which patients will respond to which drugs.
“Every pancreatic cancer is different and performing molecular profiling of each patient’s tumor could help determine the best treatment options,” said lead author Aatur Singhi, MD, PhD, surgical pathologist at UPMC and assistant professor of pathology at Pitt. “Rather than blindly giving patients the same chemotherapy, we want to tailor a patient’s chemo to their tumor type. A one-size-fits-all approach isn’t going to work. Therefore, we would like to make molecular profiling standard-of-care for patients with pancreatic cancer.”
Drs. Singhi and Bahary’s study characterized the genome of 3,594 pancreatic tumor samples from patients around the world, provided by collaborators at Foundation Medicine.
“We believe that this is the largest study in pancreatic cancer conducted using comprehensive genomic profiling to identify a broad set of genomic alterations, and ultimately, therapeutic targets, in this difficult-to-treat disease,” said Siraj Ali, MD, PhD, senior director of clinical development at Foundation Medicine.
Besides shrinking tumors with personalized chemotherapy, another way to increase pancreatic cancer survival rates is through increased pancreatic cysts screening, Dr. Singhi said, but the problem is that pancreatic cysts are incredibly common, and not all lead to cancer.
Previously, Dr. Singhi and colleagues developed a clinical molecular test known as PancreaSeq to evaluate common pancreatic cysts and identify which cases may progress to cancer. Now Drs. Singhi and Bahary’s newly discovered biomarkers can be added to the PancreaSeq platform, already being used by several institutions, including UPMC.
Targeting Deadly Tumors in Unborn Babies

Pioneered at UPMC at the Center for Innovative Fetal Intervention at UPMC Magee-Womens Hospital which is directed by McGowan Institute for Regenerative Medicine affiliated faculty member Stephen Emery, MD, a new technique using microcoil embolization shows early promise in treating inoperable placental tumors.
Sometimes a non-cancerous tumor can still be deadly — especially when it’s a chorangioma, a tumor that grows on the fetal side of the placenta and threatens the health of both the fetus and the mother. UPMC’s innovative application of a proven technique for shrinking tumors now offers hope for treating this rare, but serious, condition.
The new technique uses microcoil embolization to block the supply of blood to the chorangioma by strategically placing a tiny metal coil in the feeding artery to create a clot.
Proven to Shrink Tumors Elsewhere
Embolization is a minimally-invasive procedure used to stabilize aneurisms and stop hemorrhages in veins and arteries by inducing a blood clot to block flow. It is widely used to shrink tumors in the brain, liver, and other parts of the body. Dr. Emery, Associate Professor, Department of Obstetrics, Gynecology, & Reproductive Sciences, Maternal Fetal Medicine, at Magee-Womens Hospital of UPMC, was inspired to apply the technique in a new way when he was presented with an inoperable chorangioma that was endangering the health of a mother and her unborn son. “I thought, what would you do to a tumor in any other body compartment that’s basically inoperable? You would embolize it. That’s standard treatment.”
Though embolization had never been performed on a giant chorangioma, Dr. Emery reasoned that it could be used to shut off blood flow to the tumor and starve it. Since the coil would be inserted into the blood vessel through a thin catheter, the technique would be minimally invasive with respect to the placenta and uterus.
UPMC’s Center for Innovative Fetal Intervention had the resources to support the procedure. “I talked with our interventional radiologist, and he agreed,” says Dr. Emery. “He has years of experience embolizing tumors and I have years of experience accessing fetal blood vessels. Together, we had the experience needed to perform the operation.”
Restoring Blood and Oxygen to the Fetus
When Dr. Emery and the UPMC team performed the procedure, the microcoil embolization stopped the blood flow instantly, killing the tumor while leaving the placenta intact, and restoring normal blood and oxygen flow to the fetus. The fetus recovered, the pregnancy continued, and the mother delivered a healthy baby boy.
“People have tried all sorts of ways of doing this using fetoscopes and bipolar cautery, with really mixed results,” says Dr. Emery. “This is the first time where it was a very, very minimally invasive procedure, extremely safe for mom, with a successful outcome.”
Lower Risk, Better Odds
With these promising results, Dr. Emery sees value to the continued use of the technique. Although they are rare, life-threatening chorangiomas can occur in patients who live far from advanced fetal care centers like UPMC. “One of the benefits of this technique is that you can do it just about anywhere — you don’t need elaborate facilities,” explains Dr. Emery. “There’s minimal anesthesia and minimal trauma involved. Because it’s so low risk, you can perform it earlier in the pregnancy, when the odds of a positive outcome are better.”
The Center for Innovative Fetal Intervention at UPMC
Pioneering ways to save new lives is central to the mission of UPMC’s Center for Innovative Fetal Intervention. Founded by Dr. Emery in 2007, the center brings together the powerful resources of UPMC Magee-Womens Hospital, the University of Pittsburgh McGowan Institute for Regenerative Medicine, UPMC Children’s Hospital of Pittsburgh, and Magee-Womens Research Institute to pioneer new, cutting-edge techniques to share with the global medical community. As a member of the North American Fetal Therapy Network, a consortium of 35 academic centers in the United States and Canada that will share the research, the UPMC Center for Innovative Fetal Intervention is at the forefront of advancing the science of saving very tiny, very fragile lives worldwide.
Pitt Bioengineers Create Ultra-Small, Light-Activated Electrode for Neural Stimulation

Neural stimulation is a developing technology that has beneficial therapeutic effects in neurological disorders, such as Parkinson’s disease. While many advancements have been made, the implanted devices deteriorate over time and cause scarring in neural tissue. In a recently published paper, McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, an assistant professor of bioengineering in the University of Pittsburgh Swanson School of Engineering, detailed a less invasive method of stimulation that would use an untethered, ultra-small electrode activated by light, a technique that may mitigate damage done by current methods.
“Typically with neural stimulation, in order to maintain the connection between mind and machine, there is a transcutaneous cable from the implanted electrode inside of the brain to a controller outside of the body,” said Dr. Kozai. “Movement of the brain or this tether leads to inflammation, scarring, and other negative side effects. We hope to reduce some of the damage by replacing this large cable with long wavelength light and an ultra-small, untethered electrode.”
Kaylene Stocking, a senior bioengineering and computer engineering student, was first author on the paper titled, “Intracortical neural stimulation with untethered, ultra-small carbon fiber electrodes mediated by the photoelectric effect” (DOI: 10.1109/TBME.2018.2889832). She works with Dr. Kozai’s group – the Bionic Lab – to investigate how researchers can improve the longevity of neural implant technology. This work was done in collaboration with Alberto Vasquez, PhD, research associate professor of radiology and bioengineering at Pitt.
The photoelectric effect is when a particle of light, or a photon, hits an object and causes a local change in the electrical potential. Dr. Kozai’s group discovered its advantages while performing other imaging research. Based on Einstein’s 1905 publication on this effect, they expected to see electrical photocurrents only at ultraviolet wavelengths (high energy photons), but they experienced something different.
“We’ve had numerous critics who did not have faith in the mathematical modifications that were made to Einstein’s original photoelectric equation, but we believed in the approach and even filed a patent application” (patent pending:US20170326381A1), said Dr. Kozai. “This is a testament to Kaylene’s hard work and diligence to take a theory and turn it into a well-controlled validation of the technology.”
Dr. Kozai’s group is currently looking further into other opportunities to advance this technology, including reaching deeper tissue and wireless drug delivery.
Ms. Stocking anticipates graduating in April 2019 and plans to pursue a doctoral degree. She said, “The University of Pittsburgh has amazing resources that have allowed me to gain meaningful research experience as an undergraduate, and I’m grateful to Dr. Kozai and the Department of Bioengineering for giving me the opportunity to do impactful work.”
Illustration: A laser shining onto an untethered, ultra-small carbon fiber electrode to stimulate neurons via the photoelectric effect. Photo credit: J. Mater. Chem. B, 2015,3, 4965-4978. Reproduced by permission of The Royal Society of Chemistry.
New Hope for Heart Transplants

If you follow transplant news, you probably know the grim facts: Worldwide, there is an enormous and widening gap between the number of people who need transplant organs and the number of donor organs available. According to the United Network for Organ Sharing (UNOS), 20 people die every day waiting for a transplant. While UNOS and other organizations work to increase awareness and recruit more donors, researchers and doctors are working hard to find medical solutions that can help close that gap.
One of these researchers is McGowan Institute for Regenerative Medicine affiliated faculty member and UPMC heart transplant surgeon Christopher Sciortino, MD, PhD. He has a frontline view of the donor gap, and a passionate vision of the lives that can be saved when that gap narrows, the pool expands, and transplants take place earlier when patients have a better chance for a healthy life.
That’s why Dr. Sciortino, Assistant Professor of Cardiothoracic Surgery at the University of Pittsburgh School of Medicine and Surgical Director of the UPMC Advanced Heart Failure Center, is leading a clinical trial at UPMC that is pushing the donor envelope. The new source of hearts is one that you might not expect: donors who test positive for hepatitis C.
Hep C: Not the Threat It Once Was
Most people know hep C is a serious blood-borne viral disease that can cause liver damage, liver cancer, and death. It is transmitted through IV drug use, tattooing, and other ways that blood can pass from one person to another — including through transplants.
Although the risk of infection is only around 1 percent, until recently organs from hep C-antibody positive donors were excluded from the transplant pool. But that picture has changed for the better with the development of new antiviral hep C drugs offering a 95 percent cure rate and that are much better tolerated by immunosuppressed transplant recipients. Progressive hospitals such as UPMC, with its long history of pioneering transplant research, are leading the way in using this breakthrough to access a larger pool of donor organs, beginning with hearts.
Emphasis on Safety
Last year, under Dr. Sciortino’s leadership, UPMC embarked on a conservative, safety-focused protocol for hep C-antibody positive heart transplants. Initial criteria excluded high-risk recipients, such as dialysis patients, who are likely to suffer complications. In addition, donor organs were restricted to a four-hour maximum travel distance. “We wanted to demonstrate a foundation of safety for the process before opening it up to everyone,” says Dr. Sciortino.
The protocol has resulted in a handful of transplants over the past year — all successful operations. With these promising results, UPMC expanded its criteria to accept higher risk patients and hearts from farther away. In addition to hearts, the program now accepts kidneys, lungs, and even livers from hep C positive donors. The donor pool, which had initially been restricted to organs from donors with hep C antibodies but no disease, now includes organs from hep C positive donors.
A Natural Fit
New developments like this aren’t going to close the transplant gap any time soon, but a mainstream protocol for hep C hearts is a promising step. And it’s a natural fit for UPMC, where organ transplantation first made great strides through the pioneering research of Thomas E. Starzl, MD, in the 1980s. Today, UPMC is one of only a handful of hospitals with the resources and experience to perform hep C organ transplants. “We are a progressive center,” says Dr. Sciortino. “We don’t want to have to say to someone who needs a transplant, ‘you’re too sick for us to help you.’ Built on a foundation of safety, our approach allows us to partner with patients with challenging conditions. That’s what this work is all about.”
Penn Team Eradicates Hepatitis C in Nine Patients Following Lifesaving Heart Transplants from Infected Donors

Nine patients at Penn Medicine have been cured of the Hepatitis C virus (HCV) following lifesaving heart transplants from deceased donors who were infected with the disease, according to a study published in the American Journal of Transplantation. The results highlight the potential for expanding the use of HCV-infected organs, including hearts, to broaden the donor pool for the more than 100,000 Americans currently on a transplant waitlist. McGowan Institute for Regenerative Medicine affiliated faculty member Christian Bermudez, MD, Director of Thoracic Transplantation at the University of Pennsylvania and Associate Professor of Surgery at the University of Pennsylvania School of Medicine, was a team member on the study.
In 2017, Penn Medicine launched a clinical trial to test the effect of transplanting hearts from donors with HCV into patients on the transplant waitlist who do not have the virus. Researchers modeled the clinical trial, known as USHER, after an innovative Penn Medicine-led study that involved transplanting HCV-infected kidneys (known as THINKER), and then treating the recipients with an antiviral therapy to eradicate the virus after transplantation. In both studies, all the patients who completed the antiviral therapy regimen have been cured of their contracted HCV.
“For decades, most hepatitis C-infected hearts were often discarded – and the few people who received these organs were found to have a significantly lower rate of survival,” said Peter Reese, MD, MSCE, an associate professor of Medicine and Epidemiology, Penn Medicine. “Our trial provides fresh evidence to show that new antiviral treatments for HCV work well in immunosuppressed patients, which has the potential to really impact the field of transplantation. These preliminary results suggest that we should make it a priority to expand the use of good-quality HCV-infected organs.”
The researchers noted that this is the first trial in thoracic surgery to transplant Hepatitis C-infected hearts into Hepatitis-C negative patients with a formal protocol, which enabled detailed prospective data collection from the donor and recipient. In the case of this study, the team was able to identify novel data on the viral replication and clearance in heart recipients in the USHER trial, as well as in the kidney recipients from the team’s THINKER trial.
“Unfortunately, every year, hundreds of the nearly 4,000 people on the heart transplant waitlist either die or get too sick for transplant – a tragic problem that stems from a limited donor pool,” Dr. McLean said. “We started this trial in hopes that we could introduce an entirely new pool of donors that would significantly expand the nation’s supply of available organs, enabling us to effectively transplant hundreds more candidates. Our data suggests the use of Hepatitis C-infected hearts – when followed by antiviral therapy – can be viable option for patients who may otherwise never receive a transplant.”
The research team recently launched another new clinical trial that will study this same approach in patients who are awaiting a lung transplant. Researchers note there is a need for longer and larger trials to continue evaluating the effectiveness of HCV-positive to HCV-negative transplantation followed by antiviral therapy in a broader population.
Video: “Hope vs. Hype of Stem Cell Therapy”

This first-ever open-to-the-public session of the annual scientific retreat was organized by McGowan Institute for Regenerative Medicine faculty members based on general questions they receive from the public almost on a daily basis. The purpose of the event is to provide an honest discussion with stem cell therapy and science communication experts on what is hope, what is hype, what is experimental, and what is therapeutic relative to various advertisements and promises regarding stem cells. The program’s conversation aims to sort out the alternative facts and the fake news surrounding the medical science of stem cells. Many factors determine the safety and efficacy of stem cell treatments. Simply put, you need the right cells, to the right patient, at the right time, in the right way. If scientists do not ensure the field of stem cells research is credible through good science and ethical practice, the field will quickly become unbelievable.
“Hope vs. Hype of Stem Cell Therapy” was held at the University of Pittsburgh on March 12, 2019. Participants in the event included:
Forum Organizer:
- Bryan Brown, PhD, Assistant Professor, Department of Bioengineering, Swanson School of Engineering, University of Pittsburgh.
Moderator:
- Bill Flanagan, Chief Corporate Relations Officer, Allegheny Conference.
Panel Members:
- Carl Kurlander, Documentarian and Filmmaker; Visiting Distinguished Senior Lecturer, Faculty Adviser, Pitt In Hollywood, University of Pittsburgh.
- Peter Rubin, MD, FACS, UPMC Endowed Professor and Chair of Plastic Surgery; Director, UPMC Wound Healing Services; Professor of Bioengineering, University of Pittsburgh.
- Lawrence R. Wechsler, MD, Henry B. Higman Professor and Chair, Department of Neurology, University of Pittsburgh School of Medicine; Co-Director of The Neurological Institute; and Vice President of Telemedicine Services, UPMC.
Watch the video here.
Important Upcoming Dates
Regenerative Medicine Summer School
Registration is now open!
Click Here for more information
Pittsburgh Life Sciences Week: May 13-17, 2019
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#194 –– Dr. Bryan Brown discusses the upcoming sixth annual summer school for undergraduates and – new this year – sessions for teachers, and internships.
#195 –– A live recording of the public panel discussion held March 12, 2019: Hope vs. Hype of Stem Cell Therapies Open Session.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Singhi AD, George B, Greenbowe JR, Chung J, Suh J, Maitra A, Klempner SJ, Hendifar A, Milind JM, Golan T, Brand RE, Zureikat AH, Roy S, Schrock AB, Miller VA, Ross JS, Ali SM, Bahary N
Title: Real-time Targeted Genome Profile Analysis of Pancreatic Ductal Adenocarcinomas Identifies Genetic Alterations that Might be Targeted with Existing Drugs or Used as Biomarkers
Summary: BACKGROUND AND AIMS: It has been a challenge to select treatment for patients with pancreatic ductal adenocarcinomas (PDACs) based on genome alterations. We performed targeted genomic profile analyses of a large number of PDACs to assess the full spectrum of actionable genomic alterations METHODS: We performed targeted genomic profile analyses of 3594 PDAC samples from an international cohort, including capture-based targeted genomic profiling of as many as 315 cancer-associated genes and intron regions of 28 genes that are rearranged in cancer cells. Tumor mutation burden (TMB) and microsatellite instability (MSI) status were also assessed. TMB was calculated across a 1.14 Mb region; TMB-high (TMB-H) was defined as ≥20 mutations/Mb. MSI-high (MSI-H) status was assigned based on analysis of 114 intron homopolymer loci.
RESULTS: KRAS, TP53, CDKN2A, and SMAD4 were the most frequently altered genes in the PDAC tissues. We found KRAS mutations in 88% of samples. Among PDACs without mutations in KRAS, we found alterations in genes whose products are in the mitogen-activated protein kinase signaling pathway and are candidate drug targets (actionable targets; n=132; 4%), as well as gene fusions (n=51), gene amplifications (n=35), genes with missense mutations (n=30), and genes that contain deletions (n=16). Many of these encode proteins in receptor tyrosine kinase, RAS, or mitogen-activated protein kinase signaling pathways. Aside from TP53, alterations in genes encoding DNA damage repair proteins (BRCA and FANC) were detected in in 14% of PDACs. Among PDACs evaluated for MSI (n=2563) and TMB (n=1021), MSI-H and/or TMB-H phenotypes were detected in 0.5% of samples. Alterations in FGF23, CCND2, PIK3CA, and FGF6 were more commonly detected in intraductal papillary mucinous neoplasm-associated PDACs.
CONCLUSIONS: In targeted genomic profile analyses of 3594 PDACs, we found 17% to contain genomic alterations that might make the tumor cells susceptible to currently used anti-cancer agents. We identified mutations in genes that could contribute to progression of intraductal papillary mucinous neoplasms into malignancies. These alterations might be used as biomarkers for early detection.
Source: Gastroenterology. 2019 Mar 2. pii: S0016-5085(19)32505-3. doi: 10.1053/j.gastro.2019.02.037. [Epub ahead of print]
GRANT OF THE MONTH
PI: Marc Simon
Title: A Phase II Trial of Metformin for Pulmonary Hypertension in Heart Failure with Preserved Ejection Fraction
Description: There are several criteria by which the success or failure of implantable materials can be measured; but, without question, the manner in which the host responds to the implanted material will be a critical determinant of outcome. Largely, the interaction of immune cells with implanted materials has been considered a precursor to the foreign body reaction with associated negative impacts upon functionality. Recently, the role of the innate immune system, particularly that of macrophages, in the host response to biomaterials has received renewed attention. It has now been shown that macrophages, depending upon highly plastic and context- dependent polarization profiles (i.e. M1 pro-inflammatory vs. M2 anti-inflammatory/regulatory), are also capable of affecting positive outcomes following biomaterial implantation. This emerging understanding of the essential constructive and regulatory roles of macrophages in positive outcomes represents a significant departure from the classical paradigms of host biomaterial interactions. It now appears desirable that emerging biomaterials-based approaches to tissue reconstruction should not only accommodate but also promote involvement of the immune system to facilitate positive outcomes. However, such approaches cannot be developed without a detailed understanding of both the contributions of individual macrophage subtypes to tissue remodeling and integration and the context in which host encounters the implant. The present proposal seeks to develop a comprehensive and integrated approach to broadening the current understanding of how macrophages, their functional subsets, and host characteristics affect the host response to biomaterials. The proposed work builds upon our previous studies demonstrating that macrophage M1/M2 polarization at early time points is predictive of downstream integration outcomes and that the implantation microenvironment (i.e. tissue type, age, disease status) strongly affects the host response and subsequent tissue remodeling outcomes. Additionally, we have developed a number of model systems for assessing the specific contributions of M1 and M2 macrophages in the host response to biomaterials and novel methods for the modulation of the host response at the biomaterial surface. Collectively, these studies will provide crucial insight into the use of biomaterials as implants and provide methods for promoting improved outcomes associated with their use.
Source: National Institute on Aging
Term: March 1, 2019 – February 29, 2020
Amount: $664,604
