March 2016 | VOL. 15, NO. 3| www.McGowan.pitt.edu
McGowan Institute for Regenerative Medicine Holds Its Annual Scientific Retreat
The McGowan Institute for Regenerative Medicine held its 2016 Scientific Retreat March 6-8, 2016. The focus was on peer-to-peer networking, and the retreat provided many opportunities to explore collaborative endeavors with other researchers, participating guests, and external partners who are working to bring regenerative medicine technologies to clinical use.
The participation and contributions of the guests and external collaborators – along with McGowan Institute for Regenerative Medicine affiliated faculty and trainees – provided for insightful discussions and identification of opportunities for partnership. This year’s program was co-chaired by Fabrisia Ambrosio, PhD, MPT, Assistant Professor in the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh, and Vijay Gorantla, MD, PhD, McGowan Institute Deputy Director, Associate Professor of Surgery in the Department of Plastic Surgery, and the Administrative Medical Director of the Pittsburgh Reconstructive Transplant Program at UPMC.
Program Highlights
 The Retreat’s Opening Session was led by McGowan Institute Director William Wagner, PhD, Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. Following Dr. Wagner’s welcome, the main program for the evening was a “Tribute to the Artificial Heart Program” which included contributions from McGowan Institute faculty members—Harvey Borovetz, PhD, Distinguished Professor and former Chair (2002-2013) in the Department of Bioengineering, Swanson School of Engineering at the University of Pittsburgh, the Robert L. Hardesty Professor in the Department of Surgery, University of Pittsburgh School of Medicine, a Professor of Chemical and Petroleum Engineering and of the Clinical and Translational Science Institute, and a Deputy Director of Artificial Organs and Medical Devices at the McGowan Institute, Robert Kormos, MD, Professor with tenure specializing in cardiothoracic surgery at the University of Pittsburgh, the Director of UPMC’s Artificial Heart Program, and Co-Director of the Heart Transplantation Program, and Peter Wearden, MD, PhD, Department Chair, Division of Cardiology, Department of Cardiovascular Services, Nemours Children’s Hospital, Orlando, Florida.
The Retreat’s Opening Session was led by McGowan Institute Director William Wagner, PhD, Professor of Surgery, Bioengineering, and Chemical Engineering at the University of Pittsburgh. Following Dr. Wagner’s welcome, the main program for the evening was a “Tribute to the Artificial Heart Program” which included contributions from McGowan Institute faculty members—Harvey Borovetz, PhD, Distinguished Professor and former Chair (2002-2013) in the Department of Bioengineering, Swanson School of Engineering at the University of Pittsburgh, the Robert L. Hardesty Professor in the Department of Surgery, University of Pittsburgh School of Medicine, a Professor of Chemical and Petroleum Engineering and of the Clinical and Translational Science Institute, and a Deputy Director of Artificial Organs and Medical Devices at the McGowan Institute, Robert Kormos, MD, Professor with tenure specializing in cardiothoracic surgery at the University of Pittsburgh, the Director of UPMC’s Artificial Heart Program, and Co-Director of the Heart Transplantation Program, and Peter Wearden, MD, PhD, Department Chair, Division of Cardiology, Department of Cardiovascular Services, Nemours Children’s Hospital, Orlando, Florida.
Prior to the opening program, the retreat included several Short Courses:
- Supporting Science Reproducibility and Rigor with Research: Quality Assurance for Academic Environments
- The Innovation Institute –We Are Here to Help You….Commercialize
- Pediatric Device Development –‘Making the Connection’
- Strategies for Winning Department of Defense (DoD) Funding
A full list of the presenters in each session is available here.
 The Retreat program also included a great cross-section of scientific presentations given by invited researchers from other institutions and agencies who are not formally affiliated with the Institute (in alphabetical order):
The Retreat program also included a great cross-section of scientific presentations given by invited researchers from other institutions and agencies who are not formally affiliated with the Institute (in alphabetical order):
- Katelyn Fleishman Allison, PhD, ACSM-HFS, Assistant Professor, Neuromuscular Research Laboratory (NMRL), University of Pittsburgh: “Musculoskeletal, Biomechanical, and Physiological Gender Differences in the U.S. Military”
- Gary An, MD, Associate Professor of Surgery, Department of Surgery, University of Chicago Medicine: “Theory-Driven Biology: The Real Path Towards “Precision Medicine”
- Elisabeth Barton, PhD, Professor, College of Health & Human Performance, Applied Physiology & Kinesiology, University of Florida: “Strategies for Improving Skeletal Muscle Repair”
- Charlie Batch, Founder, Best of the Batch Foundation, Co-Founder, Impellia, Former NFL Quarterback, Vice President, Executive Committee, NFL Players Association: “PIVOTing to Market: The Importance of Academia/Industry Partnerships”
- Partha Biswas, BVSc, MVSc, PhD, Assistant Professor, Division of Rheumatology & Clinical Immunology, Department of Medicine, University of Pittsburgh: “IL-17 signaling in Kidney: Two Sides of the Same Coin”
- DaTren Chou, PhD, Swanson School of Engineering: “3-D printing & Metallic Biomaterials”
- George Christ, PhD, Professor of Biomedical Engineering and Orthopaedic Surgery, Mary Muilenburg Stamp Professor of Orthopaedic Research, Director of Basic and Translational Research in Orthopaedic Surgery, Head, Laboratory of Regenerative Therapeutics, University of Virginia: “Enabling Technologies for Muscle Repair”
- Sydney R. Coleman, MD, TribeCa Plastic Surgery: “Adipose Based Soft Tissue Regeneration for Trauma”
- Tarek Fahmy, PhD, Associate Professor of Biomedical Engineering and Immunobiology, Yale University: “Nanobiosensors and Nanoscale Applications in Transplantation”
- Zev Gartner, PhD, Associate Professor, School of Pharmacy Department of Pharmaceutical Chemistry, University of California San Francisco: “3D Printing Human Cells and Tissues with DNA Velcro”
- Hernando Gomez, MD, MPH, Assistant Professor, Department of Critical Care Medicine, Emergency Medicine and Clinical and Translational Research Center for Critical Care Nephrology, University of Pittsburgh Medical Center: “Sepsis Phenotyping”
- Kenton Gregory, MD, Director, Center for Regenerative Medicine, Oregon Health & Science University: “Treatment of Extremity Compartment Syndrome Using Autologous Bone Marrow Mononuclear Cells with Rehabilitation Exercises”
- Tamer Ibrahim, PhD, Associate Professor and Director of the Radio Frequency Research Facility, Departments of Bioengineering and Radiology, University of Pittsburgh: “MRI Ultra High Field Imaging in the Aging Brain”
- Stephen LeBeau, President, nanoMAG, LLC: “Magnesium Devices for Dental and Orthopedic Applications”
- Nathan K. LeBrasseur, MS, PhD, Associate Professor and Co-Chair of Research, Department of Physical Medicine and Rehabilitation, Mayo Clinic: “Cellular Senescence: At the Crossroads of Aging and Rejuvenation”
- Wayne Matheny, Jr., PhD, Research Physiologist, Military Performance Division, U.S. Army Research Institute of Environmental Medicine: “Mechanisms of Musculoskeletal Injury in Warfighters”
- Rudy A. Mazzocchi, CEO, Elenza, Inc. – Florida: “Trials and Triumphs of an Entrepreneur’s Journey”
- Ellen Mitchell, MD, Clinical Assistant Professor of Ophthalmology, Children’s Hospital of Pittsburgh of UPMC, Fox Center for Vision Restoration: “BrainPort and Sensory Substitution in Pediatric Patients”
- Alison Morris, MD, MS, Vice Chair of Clinical Research, Department of Medicine, Associate Professor of Medicine and Immunology, Division of Pulmonary, Allergy & Critical Care Medicine: “The Human Microbiome: Beyond the Person in Personalized Medicine”
- Volker Musahl, MD, Associate Professor, Departments of Orthopedics and Bioengineering, University of Pittsburgh, Medical Director, UPMC Center for Sports Medicine, South Side: “PIVOTing to Market: The Importance of Academia/Industry Partnerships”
- Rema Padman, PhD, Professor of Management Science & Healthcare Informatics, The H. John Heinz III College, Carnegie Mellon University: “Predictions with Clinical Data: Disease Risk, Progression and Pathway Analysis”
- Jaques Reifman, PhD, Senior Research Scientist (Advanced Medical Technology), U.S. Army Medical Research and Materiel Command, Director, Biotechnology HPC Software Applications Institute, Fort Detrick, Maryland: “Can We Predict Cognitive Performance Decrements Due to Sleep Loss and the Recuperative Effects of Caffeine?”
- Dietrich Stephan, PhD, Professor and Chairman of Human Genetics, University of Pittsburgh: “Translational Research in Precision Medicine”
- Sylvia E. Szentpetery, MD, Pediatric Pulmonology Fellow, Children’s Hospital of Pittsburgh of UPMC: “Non-Invasive Negative-Pressure Ventilation”
- Lawrence Wechsler, MD, Henry B. Higman Professor of Neurology/Neurosurgery, Chair, Department of Neurology; Vice President, Telemedicine, Physician Services Division, UPMC: “Combining Cell Therapy and Rehabilitation in Stroke Clinical Trials”
See the full Retreat Program here.
New this year, a student trainee made an invited presentation during the each technical session. The identification of these presenters was made following a review of the poster session abstracts and selection sought alignment with the theme of the respective session. The trainees (in alphabetical order) included:
- Kassandra Allbright, Medical Student; Mentor: Kacey Marra, PhD: “Poloxamer Hydrogel Delivery of Adipose-derived Stem Cells for Repair of Transecting Peripheral Nerve Injury: Positive Effects on Regenerative Gene Expression Programs in Re-innervating Muscle”
- Rebecca Duffy, PhD Graduate Student; Mentor: Adam Feinberg, PhD: “Using Micropatterned Extracellular Matrix Cues to Guide Murine and Human Myotube Formation”
- Michael Durka, PhD Graduate Student; Mentor: Anne Robertson, PhD: “CFD Modeling of Cerebral Aneurysms: Hemodynamic Sensitivity to Choice of Waveform in the Elderly Population”
- James Eles, PhD Graduate Student; Mentor: Tracy Cui, PhD: “In Vivo Tracking of the Meningeal Response to Chronically Implanted Neural Electrode Arrays”
- James Fisher, MD/PhD Student; Mentors: Steven Little, PhD, and Vijay Gorantla, MD, PhD: “Biomimetic Microparticles Can Establish Dominant Tolerance in Vascularized Composite Allotransplantation via Endogenous Regulatory T Cell Enrichment”
- Luke Ziegler, Research Fellow; Mentor: Marina Kameneva, PhD: “Encapsulation of Donor Hemoglobin in Autogenic RBCs for Potential Treatment of Sickle Cell Disease”
 Poster Session
Poster Session
The poster session was effective in introducing the focus of the Retreat and interests of the faculty and the guests. Andrew Duncan, PhD, Assistant Professor in the Department of Pathology, Division of Experimental Pathology, University of Pittsburgh, and his committee organized the session and judged the posters. The winners of the poster session were:
- Cellular and Gene Therapy
Winner: Luke Ziegler
Mentor: Marina Kameneva, PhD
Title: Encapsulation of Donor Hemoglobin in Autogenic RBCs for Potential Treatment of Sickle Cell Disease
Department: Bioengineering, University of Pittsburgh
Runner-up: Evan Delgado
Mentor: Andrew Duncan, PhD
Title: MicroRNA-122 Regulates Polyploidization in the Murine Liver
Department: Pathology, University of Pittsburgh
- Computation and Modeling
 Winner: Li Ang Zhang
Winner: Li Ang Zhang
Mentor: Gilles Clermont, MD
Title: Identifying Disease Endotypes and Time of Onset from Interleukin-6 Trajectories in Septic Patients
Department: Chemical and Petroleum Engineering, University of Pittsburgh
Runner-up: Matthew Markovetz
Mentor: Robert Parker, PhD
Title: Identification and Characterization of a Defect in Liquid-Solute Transport and Junctional Integrity in Airway Epithelia with Cystic Fibrosis
Department: Chemical and Petroleum Engineering, University of Pittsburgh
- Medical Devices
Winner: Mitali Patil
Mentor: Prashant Kumta, PhD
Title: Fabrication and optimization of parameters of vertically aligned platinum wire aptasensor arrays (VAPAA) for impedimetric detection of cardiac biomarkers
Department: Bioengineering, University of Pittsburgh
Runner-up: Xin Zheng
Mentor: Tracey Cui, PhD
Title: Evaluation of a soft elastomeric electrode for intramuscular stimulation and drug release
Department: Bioengineering, University of Pittsburgh
Tissue Engineering
Winner: Timothy Jackson
Mentor: Lance Davidson, PhD
Title: Heart progenitor cells undergo a mechanically-regulated mesenchymal-to-epithelial transition that is required for vertebrate heart formation
Department: Bioengineering, University of Pittsburgh
Runner-up: Deepthi Vijayraghavan
Mentor: Lance Davidson, PhD
Title: Mechanical Regulation of Cell Behaviors During Convergent Extension of the Xenopus Laevis Neural Plate
Department: Bioengineering, University of Pittsburgh
 In addition, the Cellular Approaches to Tissue Engineering and Regeneration (CATER) Program recognized its students. The director of the CATER Program is Satdarshan P. Singh Monga, MD, Endowed Research Chair in Experimental Pathology and Vice-Chair of the Division of Experimental Pathology, Department of Pathology, University of Pittsburgh, Professor of Pathology (Division of Cellular & Molecular Pathology), and Professor of Medicine (Division of Gastroenterology, Hepatology & Nutrition), University of Pittsburgh School of Medicine. The winners of this year’s CATER scientific poster contest were:
In addition, the Cellular Approaches to Tissue Engineering and Regeneration (CATER) Program recognized its students. The director of the CATER Program is Satdarshan P. Singh Monga, MD, Endowed Research Chair in Experimental Pathology and Vice-Chair of the Division of Experimental Pathology, Department of Pathology, University of Pittsburgh, Professor of Pathology (Division of Cellular & Molecular Pathology), and Professor of Medicine (Division of Gastroenterology, Hepatology & Nutrition), University of Pittsburgh School of Medicine. The winners of this year’s CATER scientific poster contest were:
First Place (tied): Lindsay Sadlin
Title: Understanding Esophageal Adenocarcinoma Progression Using Inflammatory and Neoplastic Extracellular Matrix Hydrogels
First Place (tied): Jacqueline Russell
Title: Lack of Beta-Catenin in Hepatocytes Impairs Proliferation and Leads to Increased Morbidity in Response to a Choline-Deficient Ethionine-Supplemented Diet
Second Place: ‘Abby’ Elizabeth C. Stahl
Title: Kupffer Cell Subsets Differ Between Young and Aged Murine Livers
Exhibitors
Seven exhibitors were present during the Retreat to promote opportunities within their realm of expertise. Exhibitors included:
- Cyfuse Biomedical K.K.
- Louis J. Fox Center for Vision Restoration
- McGowan Institute Histology Laboratory
- The Coulter Translational Research Partners II Program
- The Alliance for Regenerative Rehabilitation Research and Training (AR3T)
- S. Military Medical Recruiters
- McGowan Institute Flow Cytometry Laboratory
Retreat participants were entered into a raffle after visiting the exhibitor stations. Winners of the prizes were Peng Zhang, Xin ‘Sally’ Zheng, Aimon Iftikhar, and Firuz Feturi.
A special thank you is extended to all who made this year’s Retreat a success!
RESOURCES AT THE MCGOWAN INSTITUTE
April Special at the Histology Lab
April Showers mean big savings at the McGowan Histology Core Lab.
We would like to thank everyone who visited the Histology booth at the McGowan Retreat this year. It was so fun to meet fans of our work, and hopefully we will see a few new faces around the lab this year!
Break out your umbrellas as April is raining down savings on you lucky coupon holders! In addition to the coupons given out at the retreat, I’d like to offer 20% off our Herovici Stain:
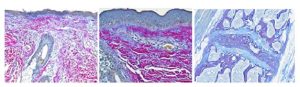
Used to differentiate young and mature collagen as well as stain reticulum. Herovici’s solution stains young collagen and reticulum blue and mature collagen red while providing a yellow cytoplasm counterstain. Nuclei are stained blue to black with Weigert’s Hematoxylin.
You’ll receive 20% off Herovici Staining for the entire month of April when you mention this ad.
Contact Lori at the McGowan Core Histology Lab by email: perezl@upmc.edu or call 412-624-5265.
As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample?
Contact Lori to find out how!
Flow Cytometry Trivia
 Flow Cytometry is a valuable technology available at the McGowan Institute for Regenerative Medicine that enables University of Pittsburgh researchers to investigate and/or characterize cells based on surface or intracellular expression of antigens, proteins, DNA, or enzyme function. Using fluorescent dyes and antibodies conjugated to fluorescent stains, the technology is able to rapidly interrogate and analyze thousands of cells per second and provide comprehensive data for each individual cell.
Flow Cytometry is a valuable technology available at the McGowan Institute for Regenerative Medicine that enables University of Pittsburgh researchers to investigate and/or characterize cells based on surface or intracellular expression of antigens, proteins, DNA, or enzyme function. Using fluorescent dyes and antibodies conjugated to fluorescent stains, the technology is able to rapidly interrogate and analyze thousands of cells per second and provide comprehensive data for each individual cell.
Just how well do you know Flow Cytometry? Try your hand at this trivia quiz.
- Which of the following CANNOT be done by Flow Cytometry:
- Analyze cell cycle profile
- Purify subpopulations by sorting
- Wash dishes
- Quantify secreted proteins
- Analyze RNA expression
- Monitor fermentation process in brewing
- Analyze cell surface markers and transcription factors
- In Flow Cytometry, compensation controls must be used for multi-color experiments:
- Always
- Never
- Only when you feel like it
- True or False: A negative control is never necessary when designing a Flow Cytometry experiment.
- True or False: Flow Cytometry can analyze multiple parameters at high rates of speed for each cell within the sample.
- Antibody titration will:
- Improve signal resolution
- Decrease non-specific binding and high background by eliminating excess antibody
- Lower costs by decreasing the quantity of antibody used
- Waste time
- All of the above except d
For the correct answers to this quiz, please contact Lynda Guzik, the Flow Cytometry Lab Manager, at 412-648-8660 or guzilj@upmc.edu. Ms. Guzik is also available to assist you in the understanding, development, and usage of multi-parameter Flow Cytometry in order to further your research.
UPCOMING EVENTS
Save the Date: SmartFlare Seminar
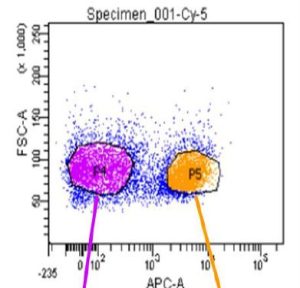
The Flow Cytometry Facility is sponsoring a Lunch and Learn Seminar Series. The next seminar will occur on Tuesday, May 3, 2016 at 12:00 – 1:30pm in Conference Room 402 at 450 Technology Drive, Bridgeside Point II. The seminar is titled “Detect RNA the smart way with SmartFlare New Technology for Live Cell RNA Detection and Biomarker Profiling” and will be presented by Matt Mandeville, Application Scientist.
SmartFlare probes are capable of detecting specific mRNA and microRNA in live cells. This technology allows for carrier-free uptake of the reagent, followed by detection of target RNA at the single cell level, with the ability to perform downstream analysis in the same sample. The reagent has no toxic effect on cells and we have demonstrated the ability to isolate and further propagate live cell populations based on gene expression levels.
For more information or to RSVP, please contact Lynda Guzik.
Save the Date: 9th Symposium on Biologic Scaffolds for Regenerative Medicine
The 9th Symposium on Biologic Scaffolds for Regenerative Medicine will be held at the Silverado Resort in Napa, California on April 28th – 30th, 2016.

Although all topic areas are considered and will be represented, the 2016 symposium will definitely include the following:
- An in depth account of the most recent findings regarding the mechanisms by which biologic scaffolds facilitate constructive remodeling of tissues; including body wall (skeletal muscle), cardiovascular, reconstructive surgical applications such as breast and pelvic floor, and whole organs such as liver, lung and heart.
- Identification and discussion of manufacturing issues such as tissue source, decellularization methods, and sterilization decisions that affect the quality and performance of biologic scaffolds for surgical applications.
- A review of clinical experiences, especially general surgery, orthopedic and trauma related challenges, neurologic applications, gastrointestinal applications, and cardiovascular applications.
- Identification and discussion of the effect of the host innate immune response upon scaffold remodeling and clinical outcome.
List of invited (and accepted) speakers to date: Robert M. Nerem, PhD (Georgia Institute of Technology), Arnold I. Caplan, PhD (Case Western Reserve University), Jeffrey M. Davidson, Ph.D. (Vanderbilt University), Cyrus Ghajar, PhD (Fred Hutchinson Cancer Research Center), Jeffrey A. Hubbell, PhD (Swiss Federal Institute of Technology, EPFL), Kristen Jones, MD (University of Minnesota), C. James Kirkpatrick MD PhD DSc FRCPath (Johannes Gutenberg University), Robert G. Martindale, MD, PhD (Oregon Health & Science University), Charles D. Mills, PhD (BioMedical Consultants), Laura E Niklason PhD, MD (Yale University), Frederick J. Schoen, MD, PhD (Harvard University), Allan S. Stewart, MD (Mount Sinai Hospital NYC), and Nadia Rosenthal, PhD (Monash University, Australia).
Save the Date: Regenerative Rehabilitation Advanced Training Course
 The University of Pittsburgh and Magee-Womens Research Institute is hosting the NIH-sponsored Rehabilitative and Regenerative Medicine for Minority Health and Health Disparities Advanced Training Course from June 6-10, 2016. The purpose of this NICHD Continuing Education Training Program is to provide comprehensive and sophisticated training and state-of-the-art methods on bioengineering, cellular, molecular and genetic approaches for advancing the fields of Rehabilitative and Regenerative Medicine. Program organizers include Drs. Gerald Schatten, Fabrisia Ambrosio, and Michael Boninger. Researchers and clinicians from around the country have been selected to present during this week-long course, including Pioneer Award Lecturer, Dr. Donald Ingber from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
The University of Pittsburgh and Magee-Womens Research Institute is hosting the NIH-sponsored Rehabilitative and Regenerative Medicine for Minority Health and Health Disparities Advanced Training Course from June 6-10, 2016. The purpose of this NICHD Continuing Education Training Program is to provide comprehensive and sophisticated training and state-of-the-art methods on bioengineering, cellular, molecular and genetic approaches for advancing the fields of Rehabilitative and Regenerative Medicine. Program organizers include Drs. Gerald Schatten, Fabrisia Ambrosio, and Michael Boninger. Researchers and clinicians from around the country have been selected to present during this week-long course, including Pioneer Award Lecturer, Dr. Donald Ingber from the Wyss Institute for Biologically Inspired Engineering at Harvard University.
This dynamic training course provides a series of daily lecturers on emerging concepts, followed by extended discussion, laboratory research, technologically intense workshops and informal seminars over a week-long period. The primary aim is to educate and update rehabilitation and regeneration researchers on the implications of stem cells and tissue engineering for mechanistic discoveries and on designing improved strategies for rehabilitation discoveries, especially in the CNS and skeletal-muscular systems. This course provides an innovative educational opportunity to motivate biomedical trainees to pursue scientific careers in rehabilitation and regenerative research research.
The target audience is young and promising faculty, as well as advanced postdoctoral fellows eager to learn about the newest findings and controversies. We intend to give participants knowledge of laboratory techniques, career mentoring and the ethical-legal-societal impact of rehabilitative and regenerative research to greatly enhance their successful entry into this field or to strengthen their knowledge in this field.
For more information, contact pdc@mwri.magee.edu or call 412-641-2400.
Link http://www.AR3T.pitt.edu/atc
SCIENTIFIC ADVANCES
Dr. Fabrisia Ambrosio Nets Two NIH Awards
McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PhD, is an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh. She holds secondary appointments in the Departments of Physical Therapy, Orthopaedic Surgery, and Microbiology & Molecular Genetics. In addition, she is a faculty member of the neurology residency program in the Department of Physical Therapy. Recently, Dr. Ambrosio and colleagues were awarded two NIH awards. Details on each follow:
Title: Dysfunctional muscle remodeling and regeneration in environmental disease (R01 from National Institute on Environmental Health Sciences)
Total funding: $2.6m
Investigators: Fabrisia Ambrosio (PI), Aaron Barchowsky (PI), William Wagner (co-I), Antonio D’Amore (co-I), Donna Stolz (co-I)
Project Description / Abstract: Arsenic is an odorless and tasteless semi-metal that contaminates drinking water supplies from natural deposits in the earth. It is estimated that almost 3.7 million individuals in the US, and over 140 million individuals worldwide, are exposed regularly to drinking water that exceed government arsenic standards. Increasing attention has been recently paid to the declines in functional mobility resulting from chronic arsenic exposure, which we now understand to pose a significant risk for causing skeletal muscle myopathies and atrophy, impairments that are among the greatest factors contributing to declines in functional mobility and strong predictors of mortality. However, many questions of the health impacts of environmental exposures throughout life remain, including the underlying mechanisms by which exposures negatively impact the cellular microenvironment, stem cell phenotype and, ultimately, tissue maintenance and healing capacity. The objective of this proposal is to elucidate mechanisms for arsenic-stimulated alteration of transcriptional circuitries that disrupt intra- and inter-cellular communication within the niche and compromise muscle structural integrity.
We propose two specific aims to test our central hypothesis that arsenic stimulates fibroblast-mediated pathogenic matrix alterations, resulting in stem cell dysfunction and, ultimately, impaired wound repair after injury. In Specific Aim 1, we will test the hypothesis that exposure to commonly encountered levels of arsenic in drinking water promotes pathologic matrix remodeling to impair muscle stem cell function and regenerative capacity. In Specific Aim 2, we will test the hypothesis that arsenic dysregulates fibroblast activation, driving an impaired muscle stem cell function and tissue repair response after injury. Success in these aims will both increase our understanding of the pathogenesis of arsenic-induced clinical symptoms of myasthenia, and will elucidate skeletal muscle microenvironmental factors controlling stem cell declines. The long-term goal is to create prevention and/or intervention strategies to improve health outcomes of individuals living in arsenic-endemic areas.
Title: The anti-aging effect of Klotho on skeletal muscle regeneration (R56 from National Institute on Aging)
Total funding: $177k
Investigators: Fabrisia Ambrosio (PI), Bennett Van Houten (PI), Aaron Barchowsky (co-I), Mauricio Rojas (co-I), Donna Stolz (co-I)
Project Description / Abstract: An age-related impairment of the regenerative capacity of aged muscle is a major contributor to declines in functional mobility and is associated with an increased morbidity in an elderly population. Following an acute injury, young skeletal muscle initiates a highly effective regenerative response, which largely restores the original architecture of the damaged fibers. Conversely, with increasing age, the regenerative response to injury results in a considerable scar tissue deposition at the expense of functional contractile tissue. Much of this healing defect has been attributed to an age-related decrease in muscle stem, or satellite, cell (MuSC) functionality. In response to skeletal muscle injury, MuSCs become activated from a quiescent state to repair damaged myofibers. However, it has been suggested that the increased fibrosis deposition following injury is a result of a myogenic-to-fibrogenic conversion of MuSCs. Fortunately, these age-related changes are reversible.
Elegant studies employing heterochronic parabiosis, in which the circulatory systems of young and aged animals are conjoined, have revealed that rejuvenation of the systemic microenvironment significantly restores both whole tissue and MuSC regenerative capacity in aged muscle. These findings implicate that circulating factors, such as the longevity protein Klotho, play a critical role in dictating skeletal muscle regenerative potential over time. Elucidation of the origin and nature of circulating factors contributing to the aged muscle phenotype is critical for the development of strategies to prevent, delay or reverse age-related declines. These studies will evaluate the role of circulating Klotho as a key anti-geronic protein contributing to the benefit of heterochronic parabiosis on aged muscle regeneration.
In Specific Aim 1, we will test the hypothesis that aged parabionts paired with young mice heterozygously deficient for Klotho will display significantly impaired skeletal muscle regeneration when compared to aged parabionts paired with young wild type mice. In Specific Aim 2, we will test the hypothesis that restoration of circulating Klotho to youthful levels reverse age-related declines in skeletal muscle regenerative capacity. We anticipate that success in these aims will have a long and lasting impact on the field as they will establish Klotho as an important anti-geronic factor that regulates MuSC activity essential for functional muscle regeneration after injury.
Congratulations Dr. Ambrosio!
FDA: In Silico Trials for Advancing New Devices and Drug Therapy Applications

At the University of Pittsburgh, Yoram Vodovotz, PhD, is a professor in the Department of Surgery with secondary appointments in the Department of Computational & Systems Biology, the Department of Bioengineering, the Department of Immunology, the Department of Communication Science and Disorders (of the School of Health and Rehabilitation Science), and the Clinical and Translational Science Institute. He also is the director of the Center for Inflammation and Regenerative Modeling at the McGowan Institute for Regenerative Medicine. In this latter role, his research interests, carried out in the context of an interdisciplinary research team, include systems biology and mathematical modeling of inflammation and wound healing in various disease states, especially infection, trauma/hemorrhage, wound healing, and liver failure, as well as the application of these computational approaches (i.e. in silico) to regenerative medicine, biomarker discovery, and rational drug/device design. In silico study in medicine is thought to have the potential to speed the rate of discovery while reducing the need for expensive lab work and clinical trials.
Dr. Vodovotz and his colleagues have published two recent papers pertinent to in silico research. They are:
- A computational, tissue-realistic model of pressure ulcer formation in individuals with spinal cord injury. Cordelia Ziraldo, Alexey Solovyev, Ana Allegretti, Shilpa Krishnan, M. Kristi Henzel, Gwendolyn A. Sowa, David Brienza, Gary An, Qi Mi, Yoram Vodovotz. PLoS Computational Biology: 2015 Jun 25;11(6):e1004309. eCollection 2015.
- Trauma in silico: individual-specific mathematical models and virtual clinical populations. Brown D, Namas RA, Almahmoud K, Zaaqoq A, Sarkar J, Barclay DA, Yin J, Ghuma A, Abboud A, Constantine G, Nieman G, Zamora R, Chang SC, Billiar TR, Vodovotz Y. Science Translational Medicine: 2015 Apr 29;7(285):285ra61.
An in silico clinical trial is an individualized computer simulation used in the development or regulatory evaluation of a medicinal product, device, or intervention. While completely simulated clinical trials are not feasible with current technology and understanding of biology, its development is expected to have major benefits over current in vivo clinical trials, and research on it is being pursued.
Noted by the Virtual Physiological Human Institute for Integrative Biomedical Research in Belgium, regulators in the US are beginning to recognize the potential for modeling and simulation technologies to improve the delivery of safe and efficacious biomedical products. The following text is taken from the Senate Fiscal Year 2016 FDA Appropriations Bill (S. 1800) & Report (S. Rept. 114-82):
“In Silico Clinical Trials. – In silico clinical trials use computer models and simulations to develop and assess devices and drugs, including their potential risk to the public, before being tested in live clinical trials. Advanced computer modeling may also prove useful in helping to predict how a drug or device will behave when deployed in the general population or when used in particular circumstances, thereby helping to protect the public from the unintended consequences of side effects and drug interactions. In silico trials may potentially protect public health, advance personalized treatment, and be executed quickly and for a fraction of the cost of a full scale live trial. The FDA has advocated the use of such systems as an additional innovative research tool. Therefore, the Committee urges FDA to engage with device and drug sponsors to explore greater use, where appropriate, of in silico trials for advancing new devices and drug therapy applications.”
Dr. Vodovotz recognizes this is a significant step for in silico medicine and the future benefits of it.
Retinal Regeneration Is a Fishy Problem

As reported by Carrie Fogel in the Fall 2015 Sight & Sound newsletter of the Eye and Ear Foundation of Pittsburgh, while most people anticipate the summer season as a time for vacations and time away from work, that was not the case for McGowan Institute for Regenerative Medicine affiliated faculty member Jeffrey Gross, PhD, the new Director of the Louis J. Fox Center for Vision Restoration. Dr. Gross arrived in Pittsburgh in late July; a time when many were spending their days on sunny beaches, Dr. Gross was already hard at work setting up his new laboratory in the University of Pittsburgh’s Biomedical Science Tower. Dr. Gross’ research is now conducted at the Charles and Louella Snyder Laboratory for Retinal Regeneration and focuses on ocular development, disease, and regeneration.
“I had an initial phone conversation with McGowan Institute for Regenerative Medicine faculty member Joel Schuman, MD, Chairman of the Department of Ophthalmology at the University of Pittsburgh, during which we discussed the Fox Center and the collaboration of the Department of Ophthalmology with the McGowan Institute, which I found interesting.” After a few meetings with Dr. Schuman, Dr. Gross came away from each visit more enthusiastic. “I really liked that here, at Pitt, the clinicians and basic scientists can collaborate and the barrier in getting everyone to talk to each other does not exist, as it does at other institutions and it shows in the science that is being done here.” The scientists, too, left a positive impression on Dr. Gross: “It feels like the scientists and the physicians have this ‘can-do attitude.’ Everyone I talked to made me believe that if you had a research question, people are ready to jump in and work with you and it feels like the whole team wants everyone to be successful.”
In addition to the positive reactions he received from the scientists, the unique partnership that exists among University of Pittsburgh scientists, McGowan Institute scientists, and UPMC physicians was attractive to Dr. Gross as well: “It’s very collaborative here and, as someone who sees the great potential for collaboration in the direction my work is headed, I understood how beneficial it could be for my research. The science being done here is fantastic. People actively solve problems here and that helps find opportunities for collaboration as well as finding funding for projects. The hurdles that exist to doing innovative science are minimal at Pitt, and it seemed like we could really succeed here.”
The laboratory, he explains, is a basic science laboratory that focuses on understanding the diseases of the eye, why certain people get them, and developing regenerative therapies to cure them. We investigate genetics and molecular biology and use zebrafish models, because their genetic makeup is similar to that of humans and they have a remarkable ability to regenerate their retina and Retinal Pigment Epithelial (RPE) cells after injury. Additionally, any effects of the genetic changes that are made to the zebrafish model are easy to see, as larvae and embryos are transparent, meaning that ocular defects can be more easily tracked than in mammal models. As Dr. Gross explains, “We can create a mutation in a genome and identify fish that have eye diseases that resemble humans, like macular degeneration, cataracts, coloboma, and more. By understanding what proteins and compounds are present or not present, we can see how that creates a defect and thus how you create therapies to counteract that defect from occurring. You need to understand what went wrong so that you can fix it.”
As the new director of the Fox Center, Dr. Gross is focused on supporting the research already happening and growing the support base for the center. One goal he does hope to accomplish is to strengthen our capacity to fund seed projects. “The way that federal funding works now is that it’s hard to find support for really innovative new ideas. At the Fox Center, we want to foster this innovation and creativity because we believe that this is how really groundbreaking research comes about.” Dr. Gross hopes that by using donor support, which has already contributed so much, that will leverage support from the National Eye Institute, as well as other large foundations. “The research here is fantastic,” he says, “and I believe we’re well on our way to establishing ourselves nationally and internationally as THE center for regenerative ophthalmology.”
Illustration: E. Matilda Ziegler Foundation for the Blind, Inc.
Gene Therapy for Parkinson’s Disease to Be Tested by Pitt, UPMC Experts

Experts at the University of Pittsburgh School of Medicine are leading the second arm of a clinical trial using gene therapy to relieve the symptoms of tremor and mobility impairment in patients with Parkinson’s disease. The technique shows promise in prolonging the effectiveness of levo-dopa, the mainstay treatment for the progressive neurodegenerative condition, by increasing production of a key enzyme essential to convert the drug into the neurotransmitter dopamine.
An estimated 7 to 10 million people worldwide have Parkinson’s disease, which is caused by degeneration of dopamine-producing neurons, said McGowan Institute for Regenerative Medicine affiliated faculty member Mark Richardson, MD, PhD, assistant professor of neurological surgery, Pitt School of Medicine, and director of Epilepsy and Movement Disorders Surgery at UPMC. Levo-dopa can replace the deficient dopamine for a while, but eventually the drug loses effectiveness, and subsequent increases in dosage may cause disabling side effects.
“Less dopamine is made as the neurons degenerate, and one reason is that there is a decrease in an enzyme needed to turn levo-dopa into dopamine,” said Dr. Richardson, who is the principal investigator of the Pitt arm of the trial. “By inserting the gene for this enzyme into cells in a specific part of the brain, we hope to make levo-dopa treatment more effective for a longer period of time.”
In the gene therapy, a harmless virus called adeno-associated virus-2 is used to carry the gene that makes the enzyme – aromatic L-amino acid decarboxylase (AADC) – into neurons. Once in the neurons, cellular machinery kicks into gear to increase production of the AADC enzyme.
To deliver the treatment, Dr. Richardson will make a small hole in each side of the patient’s skull to insert a thin catheter into the putamen, a brain region that is an important part of the circuit affected by Parkinson’s disease. After the gene therapy is infused over several hours, the catheter is withdrawn and the skull is repaired.
Different infusion doses will be tested during the course of the study. Up to 20 participants at two sites, Pittsburgh and San Francisco, will be followed for up to 3 years to have their disease and medication status reassessed.
The principal investigator of the trial, which is sponsored by Voyager Therapeutics, is Krystof Bankiewicz, MD, PhD, of the University of California, San Francisco, who also led an earlier trial that showed the gene therapy can be safely administered.
Individuals between 40 and 70 who have had Parkinson’s disease for more than 5 years and have been taking levo-dopa for at least 3 years may be eligible to participate in the trial. For more information, contact Patricia Porter, BS, at 412-648-8983 or Porterpm2@upmc.edu.
AWARDS AND RECOGNITION
McGowan Institute Affiliated Faculty Members Receive CSC Awards for Excellence

The Carnegie Science Center (CSC) established the Awards for Excellence program in 1997 to recognize and promote outstanding science and technology achievements in Western Pennsylvania. The Carnegie Science Awards have honored the accomplishments of more than 400 committed individuals and organizations that have improved lives through their contributions in science and technology.
The 20th Annual Carnegie Science Awards celebration will be held on May 6, 2016, at Carnegie Music Hall in Oakland during which time McGowan Institute for Regenerative Medicine affiliated faculty members Prashant Kumta, PhD, and Rocky Tuan, PhD, will be honored for their tremendous work and its impact on the vitality in the region.
 Dr. Prashant Kumta will receive the Advanced Materials Award which recognizes accomplishments in materials science that create new materials or properties leading to significant business, economic, or societal benefits for the region.
Dr. Prashant Kumta will receive the Advanced Materials Award which recognizes accomplishments in materials science that create new materials or properties leading to significant business, economic, or societal benefits for the region.
Dr. Kumta holds the Edward R. Weidlein chair professor at the University of Pittsburgh Swanson School of Engineering and School of Dental Medicine and is a professor in the Departments of Bioengineering, Chemical and Petroleum Engineering, Mechanical Engineering and Materials Science, and Department of Oral Biology. He is also Engineering Director of the Center for Craniofacial Regeneration and the Founding Director of the Center for Complex Engineered Multifunctional Materials, both at the University of Pittsburgh. During his career, Dr. Kumta has made pioneering achievements in advanced materials AND in advanced manufacturing which have led to a new platform technology for significantly improving methods to repair severely damaged bone.
Dr. Kumta and his colleagues have developed a family of biodegradable materials to fix broken bones. While simple fractures are repaired with a simple plaster cast, complicated fractures require metal screws, pins, rods, or plates to hold the bone in place until the fractures heal. Currently, surgeons fix complex fractures arising from battlefield wound or traffic accident injuries using synthetic, inert materials such as titanium, stainless steel, or nondegradable polymer. The patient is forced to live with the hardware for life. It can be removed following bone healing, but that operation might cause other medical problems. The new advances described here offer the surgeon the opportunity to use biocompatible and biodegradable “fixation devices” providing the initial structural support for bone healing. At the same time, the novel magnesium/iron alloys developed by Dr. Kumta resorb into the body so that after bone healing, the plates and screws are not “lingering” in the body. The second component of this innovative solution is an injectable “bone putty” that can be injected between bone fragments serving to significantly enhance the speed and the quality of the bone healing process. Finally, through the use of a unique binder‐jet adaptive manufacturing process, these bone support components can be manufactured to closely replicate and restore the original boney structures of patients.
The impact of this platform technology nation‐wide has been significant. The Department of Defense has aggressively supported the development of these systems to aid rehabilitation of wounded warriors. Once FDA approvals for the use of bone putty and the resorbable metals are received, we anticipate seeing widespread clinical use in orthopedic and musculoskeletal settings based on the technology and the products that come from Pittsburgh. The total medical costs associated with all musculoskeletal conditions amount to nearly $215 billion/year projected to increase further due to aging of the US population. Bone fracture injuries are common worldwide occurrence with ~6 million/year in the U.S. alone, accounting for ~10 million annual hospital visits. The platform technology developed by Dr. Kumta could significantly improve the speed and the quality of the recovery.
The work by Dr. Kumta and his colleagues is revolutionary, offering the prospect of a better outcome for the patient. Implantation of the currently used state‐of‐the‐art biocompatible but inert screw or plate or joint that remains for the life of the patient leaves the patient susceptible to severe side effects. These include arthritis, debris formation, and accumulation of chemicals in various organs running the risk of cancer in patients.
On the other hand, using the revolutionary technology of Dr. Kumta, doctors will be able to use degradable metals and ceramic putties that utilize the body’s own regenerative ability to provide a more effective method to heal the injury with no deleterious side effects. There are several unique features of this overall bone repair system: Both the novel magnesium/iron alloys and the calcium phosphate putty developed by Dr. Kumta are biocompatible and are resorbable by the body. Both provide suitable mechanical strengths matching the human bone to permit full regrowth and restoration of the damaged bone allowing no room for any unwanted secondary fractures. The putty is injectable or can be manufactured in desired shapes and sizes to match the patient needs by the binder-jet adaptive manufacturing methods. Both sub‐systems can be produced in any desired configuration and macro scale structure by additive manufacturing. There are multiple future applications such as dental restoration and other conditions involving bone loss due to traumatic battlefield or civilian injuries and debilitating diseases such as osteoporosis and bone cancer.
 Dr. Rocky Tuan will receive the Life Sciences Award which recognizes and honors scientific advances in new and innovative biomedical and life sciences endeavors.
Dr. Rocky Tuan will receive the Life Sciences Award which recognizes and honors scientific advances in new and innovative biomedical and life sciences endeavors.
Dr. Tuan is the director, Center for Cellular and Molecular Engineering, the Arthur J. Rooney, Sr. professor and executive vice chair, Department of Orthopaedic Surgery, the associate director, McGowan Institute for Regenerative Medicine, the director, Center for Military Medicine Research, and a professor in the Departments of Bioengineering and Mechanical Engineering and Materials Science, University of Pittsburgh. Dr. Tuan directs a multidisciplinary research program, which focuses on orthopaedic research as a study of the biological activities that are important for the development, growth, function, and health of musculoskeletal tissues, and the utilization of this knowledge to develop technologies that will regenerate and/or restore function to diseased and damaged skeletal tissues. Ongoing research projects are directed towards multiple aspects of skeletal and related biology, including skeletal development, stem cells, growth factor signaling, bone-biomaterial interaction, extracellular matrix and cell-matrix interaction, nanotechnology, biomaterials, 3D printing, mechanobiology, regenerative medicine, and tissue engineering, utilizing an integrated experimental approach combining contemporary technologies of biochemistry, cell and molecular biology, embryology and development, cellular imaging, and engineering. He is internationally recognized as for his pioneering studies into the cause and the treatment of osteoarthritis. His studies have shown the feasibility of using 3D microphysiological systems for testing drug toxicity and efficacy.
For more than 30 years, Dr. Tuan has studied the workings of the musculoskeletal system and its diseases, including cartilage development and repair, cell signaling and matrix biochemistry, stem cell biology, nanotechnology, and other orthopaedic topics. He is well known for his innovative research on the development, growth, and function of the musculoskeletal system. He has authored more than 400 refereed publications.
He has done pioneering work on adult stem cells and cell-based tissue engineering, in particular applications in skeletal tissue engineering and repair. His research on cartilage has included cellular signaling in developmental chondrogenesis and the utilization of adult stem cells in tissue engineering. Dr. Tuan has also pioneered the development of molecular diagnostic technologies for orthopaedic infections, an achievement that has significant impact in the clinical setting. In 2004, he received the prestigious Marshal Urist Award for Excellence in Tissue Regeneration Research from the Orthopedic Research Society.
Dr. Tuan has placed special emphasis on building strong basic science foundations for the treatment of injuries and diseases of the musculoskeletal system, and utilizing nanotechnology and mechanobiological principles in combination with bioreactor technology for functional skeletal tissue engineering and regeneration. Since joining the University of Pittsburgh, Dr. Tuan has established a national and international center of excellence built on research innovation, a strong education program, and an entrepreneurial culture that fosters local and regional collaborations among the academic, industrial, and business communities. Dr. Tuan has received multiple awards for his teaching and mentoring skills, including the Outstanding Mentor Award, National Institutes of Health, and on two occasions the Special Recognition Award, National Institutes of Health Undergraduate Scholars Program. In 2014, he was named a Distinguished University Professor by University Chancellor Mark Nordenberg.
Congratulations, Drs. Kumta and Tuan!
Illustration: Carnegie Science Center.
Dr. Rocky Tuan Joins Orthocell Limited Medical and Scientific Advisory Board

Australian regenerative medicine company Orthocell Limited recently announced the appointment of regenerative medicine expert and McGowan Institute for Regenerative Medicine Associate Director Rocky Tuan, PhD, to its Medical and Scientific Advisory Board (MSAB).
The MSAB advises Orthocell on strategic matters such as clinical research and product development. It provides valuable expertise to Orthocell as it expands its unique tendon and soft tissue repair products into international markets and advances opportunities in tissue specific growth factors and laboratory manufactured human tendons.
Dr. Tuan is a widely published and respected expert in regenerative medicine and stem cells. He is currently the Director of the Center for Cellular and Molecular Engineering in the Department of Orthopaedic Surgery and Director of the Center for Military Medicine Research, within the School of Medicine, at the University of Pittsburgh.
Dr. Tuan said, “The field of regenerative medicine is advancing at a rapid pace and Orthocell is a leader in musculoskeletal regeneration with its groundbreaking tendon and soft tissue regeneration and repair products. I am excited to join the MSAB and look forward to contributing to the company’s continued development.”
Dr. Tuan’s past appointments include the National Institute of Health (NIH) as Chief of the Cartilage Biology and Orthopaedics Branch. In 2004, Dr. Tuan received the Marshall Urist Award for Excellence in Tissue Regeneration Research of the Orthopaedic Research Society.
Orthocell CEO Mr. Paul Anderson said, “We are delighted to have such an esteemed researcher as Dr. Tuan join our MSAB. His expertise in regenerative medicine is a perfect fit for the company and we’re excited to attract such an experienced and well-respected researcher in regenerative medicine from the US to advise the company moving forward.”
Congratulations, Dr. Tuan!
Dr. Robert Kormos Named 2016 Peter J. Safar Pulse of Pittsburgh Award Winner by American Heart Association

The 2016 Pittsburgh Heart Ball took place on February 20th at the Pittsburgh Wyndham Grand Hotel. This affair marks the presentation of the Peter J. Safar Pulse of Pittsburgh Award. This year the award was received by McGowan Institute for Regenerative Medicine faculty member Robert Kormos, MD (pictured with his wife, Mary Anne).
Dr. Kormos is currently a professor with tenure specializing in cardiothoracic surgery at the University of Pittsburgh. He is the director of UPMC’s Artificial Heart Program and co-director of the Heart Transplantation Program. Dr. Kormos has been on the faculty at the University of Pittsburgh since 1987 after completion of fellowships in research and transplant surgery. He is also a staff cardiothoracic surgeon at several UPMC hospitals and Pittsburgh’s Veterans Administration Hospital.
The American Heart Association – Allegheny Division board of directors and the Heart Ball committee created the Pulse of Pittsburgh Award in 2003 to recognize an individual’s leadership in the fight against heart disease and stroke. In 2004, the committee changed the name to the Peter J. Safar Pulse of Pittsburgh Award in honor of the late Peter J. Safar, MD, distinguished professor of resuscitation medicine at the University of Pittsburgh, who also was known as the Father of CPR.
As one of Pittsburgh’s premiere social and fundraising events, the Pittsburgh Heart Ball is the American Heart Association – Allegheny Division’s largest fundraising event. Seventy-five percent of contributions support research, school-site programs, and public and professional education programs in this region. Currently, the American Heart Association is funding 37 research projects totaling more than $8.5 million in the Pittsburgh area. The funding from the Heart Ball directly supports the American Heart Association’s mission of “Building healthier lives, free of cardiovascular diseases and stroke.”
Congratulations, Dr. Kormos!
Illustration: Rebecca Droke, Pittsburgh Post-Gazette.
Association of Academic Physiatrists Honors Pitt/UPMC Physician

McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD, director of the UPMC Rehabilitation Institute and professor and chair of the Department of Physical Medicine & Rehabilitation, University of Pittsburgh School of Medicine, received the 2016 Association of Academic Physiatrists (AAP) Distinguished Academician Award at the association’s annual meeting in Sacramento, California. Each year, the AAP honors one academic physiatrist who has achieved distinction and peer recognition regionally or nationally for outstanding performance in teaching, research, or administration.
“I feel very lucky to work in a field where I have the opportunity to help people. The University of Pittsburgh and UPMC have provided me the opportunity, support, and collaborators to excel in this pursuit,” Dr. Boninger said.
The author of four U.S. patents, Dr. Boninger is recognized for his extensive research on spinal cord injury and assistive technology and overuse injuries, particularly those associated with manual wheelchair propulsion.
Dr. Boninger earned his medical degree at Ohio State University. He completed residencies at St. Joseph Mercy Hospital in Ann Arbor and the University of Michigan Medical Center, where he became the chief resident, physical medicine and rehabilitation. He came to the University of Pittsburgh when he won a postdoctoral fellowship in engineering and rehabilitation technology in 1994.
The AAP Distinguished Academician Award was established in 1995. Dr. Boninger is the first faculty member from Pitt’s Department of Physical Medicine and Rehabilitation to win this national award.
Congratulations, Dr. Boninger!
SME/AIME Recognition to John Murphy

John Murphy is the 2016 recipient of the Erskine Ramsay Medal, presented by the Society for Mining, Metallurgy and Exploration (SME) and the American Institute of Mining, Metallurgical and Petroleum Engineers. He was recognized “for his unselfish, tireless contributions to the coal industry, safety and health research, public outreach, professional development, mentoring and, of course, the Society.” Prior to joining the University of Pittsburgh, Murphy served as the research director at the Pittsburgh Research Center of the U.S. Bureau of Mines from 1978 to 1997. He served as a senior scientist at the Pittsburgh Research Laboratory, National Institute for Occupational Safety and Health from 1997 to 2001. Murphy is a Distinguished Member of SME and a past president of SME (2011) and the SME Foundation (2004-2009).
AIME Honorary Membership was also presented to John Murphy “in recognition of his dedication and perseverance in developing a purpose and mission for the SME Foundation, continuing through his exemplary service as SME president.” AIME Honorary Membership is one of the highest honors that the Institute can bestow on an individual. It is awarded in appreciation of outstanding service to the Institute or in recognition of distinguished scientific or engineering achievement in the fields embracing the activities of AIME and its Member Societies.
Congratulations, Mr. Murphy!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#157 –– Dr. An is an Associate Professor of Surgery at the University of Chicago. Dr. An discusses the application of complex systems analysis to sepsis and inflammation through modeling.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
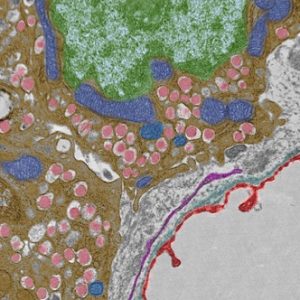
False colored pancreatic beta cells, highlighting the insulin granules (pink), mitochondria (blue), nucleus (green), fenestrated capillary (red).
Transmission Electron Microscopy. Mara Sullivan and Donna Stolz, Center for Biologic Imaging, Cell Biology.
PUBLICATION OF THE MONTH
Author: Nishikawa T, Bell A, Brooks JM, Setoyama K, Melis M, Han B, Fukumitsu K, Handa K, Tian J, Kaestner KH, Vodovotz Y, Locker J, Soto-Gutierrez A, Fox IJ.
Title: Resetting the transcription factor network reverses terminal chronic hepatic failure.
Summary: The cause of organ failure is enigmatic for many degenerative diseases, including end-stage liver disease. Here, using a CCl4-induced rat model of irreversible and fatal hepatic failure, which also exhibits terminal changes in the extracellular matrix, we demonstrated that chronic injury stably reprograms the critical balance of transcription factors and that diseased and dedifferentiated cells can be returned to normal function by re-expression of critical transcription factors, a process similar to the type of reprogramming that induces somatic cells to become pluripotent or to change their cell lineage. Forced re-expression of the transcription factor HNF4α induced expression of the other hepatocyte-expressed transcription factors; restored functionality in terminally diseased hepatocytes isolated from CCl4-treated rats; and rapidly reversed fatal liver failure in CCl4-treated animals by restoring diseased hepatocytes rather than replacing them with new hepatocytes or stem cells. Together, the results of our study indicate that disruption of the transcription factor network and cellular dedifferentiation likely mediate terminal liver failure and suggest reinstatement of this network has therapeutic potential for correcting organ failure without cell replacement.
Source: Journal of Clinical Investigation. 2015 Apr;125(4):1533-44. Epub 2015 Mar 16.
GRANT OF THE MONTH
PI: Fabrisia Ambrosio and Aaron Barchowsky
Co-PI: William Wagner, Antonio D’Amore, and Donna Stolz
Titles: Dysfunctional Muscle Remodeling and Regeneration in Environmental Disease
Description: Arsenic is an odorless and tasteless semi-metal that contaminates drinking water supplies from natural deposits in the earth. It is estimated that almost 3.7 million individuals in the US, and over 140 million individuals worldwide, are exposed regularly to drinking water that exceed government arsenic standards. Increasing attention has been recently paid to the declines in functional mobility resulting from chronic arsenic exposure, which we now understand to pose a significant risk for causing skeletal muscle myopathies and atrophy, impairments that are among the greatest factors contributing to declines in functional mobility and strong predictors of mortality. However, many questions of the health impacts of environmental exposures throughout life remain, including the underlying mechanisms by which exposures negatively impact the cellular microenvironment, stem cell phenotype and, ultimately, tissue maintenance and healing capacity. The objective of this proposal is to elucidate mechanisms for arsenic-stimulated alteration of transcriptional circuitries that disrupt intra- and inter-cellular communication within the niche and compromise muscle structural integrity.
We propose two specific aims to test our central hypothesis that arsenic stimulates fibroblast-mediated pathogenic matrix alterations, resulting in stem cell dysfunction and, ultimately, impaired wound repair after injury. In Specific Aim 1, we will test the hypothesis that exposure to commonly encountered levels of arsenic in drinking water promotes pathologic matrix remodeling to impair muscle stem cell function and regenerative capacity. In Specific Aim 2, we will test the hypothesis that arsenic dysregulates fibroblast activation, driving an impaired muscle stem cell function and tissue repair response after injury. Success in these aims will both increase our understanding of the pathogenesis of arsenic-induced clinical symptoms of myasthenia, and will elucidate skeletal muscle microenvironmental factors controlling stem cell declines. The long-term goal is to create prevention and/or intervention strategies to improve health outcomes of individuals living in arsenic-endemic areas.
Source: National Institute on Environmental Health Sciences
Term: 9/1/2015-1/31/2021
Amount: $2.6M

