July 2022 | VOL. 21, NO. 7| www.McGowan.pitt.edu
Pittsburgh Craniofacial Sciences Training Program (PCSTP)

With funding from the National Institutes of Health, National Institute of Dental & Craniofacial Research (NIDCR), the University of Pittsburgh School of Dental Medicine will establish a new Research Training Program aimed at preparing the next generation of Craniofacial/Dental focused scientists. Major research foci at Pitt Dental are Craniofacial/Dental Genetics and Craniofacial/Dental Tissue Regeneration as well as translational research. Trainees will be included at both the pre-doctoral and post-doctoral levels.
The project principal investigator is McGowan Institute for Regenerative Medicine faculty member Charles Sfeir, DDS, PhD (pictured), Associate Dean of Research in the University of Pittsburgh School of Dental Medicine, an Associate Professor in the Clinical and Translational Science Institute, an Associate Professor in the School of Dental Medicine (Departments of Oral Biology and Periodontology) and the School of Engineering (Biomedical Engineering), and the Founding Director of the Center for Craniofacial Regeneration. The project is funded for 5 years.
The project is designed to establish a new Pittsburgh Craniofacial Sciences Training Program (PCSTP) at the University of Pittsburgh School of Dental Medicine (Pitt Dental) to train and mentor a new generation of leaders in Dental Oral and Craniofacial (DOC) sciences. Our goal is to address the critical shortage of craniofacial biologists nationwide through the PCSTP. Pitt Dental is an ideal academic setting for a high-caliber program, including strong institutional investment in internationally recognized research programs in craniofacial genetics and regenerative medicine. Indeed, Pitt Dental has ranked in the top 10 dental schools receiving NIDCR funding each of the past 6 years, while the University has ranked 6th or higher nationally in total NIH funding for more than a decade. Concerted efforts to grow the research enterprise at Pitt Dental over the last 15 years led to a doubling in the number of accomplished research faculty and the completion of a new state-of-the-art research tower in 2015. The thriving Pitt Dental research ecosystem is multi- and inter-disciplinary and team-based. Our current oral and craniofacial sciences trainees work alongside PhD students from the Departments of Bioengineering (School of Engineering) and Human Genetics (Graduate School of Public Health), all of whom identify themselves as craniofacial scientists. Pitt Dental leadership fully recognizes the strategic importance of our program and has committed significant resources in personnel and support for the students in the PCSTP. The PCSTP will be administered and managed in the Department of Oral and Craniofacial Sciences, with additional faculty from other Pitt Dental or University departments (primarily Prosthodontics, Human Genetics, and Bioengineering). Trainees will be admitted to the Oral and Craniofacial Sciences PhD program and following the completion of their first year (supported by Pitt Dental), the strongest students will be considered for acceptance into the PCSTP. Postdoctoral trainees can apply to participate in either the conventional postdoctoral training or enroll in the PhD program for individuals holding a DDS/DMD. Finally, the R90 component will provide opportunities for individuals with foreign DDS/DMD degrees to participate. The PCSTP provides courses, research experiences, seminar series, journal clubs, an annual symposium, and an annual retreat (for PCSTP trainees and faculty only) and will leverage University resources, such as training in artificial intelligence, data science and the responsible conduct of research (RCR) and in methods to enhance reproducibility. Our trainees will benefit from a wealth of mentors, seminars, conferences, shared facilities, intramural pilot awards, and specialized training and experiences available through hundreds of laboratories and clinics across the six schools of the health sciences and the Pitt Clinical and Translational Sciences Institute (including its RCR Center and Institute for Clinical Research Education).
RESOURCES AT THE MCGOWAN INSTITUTE
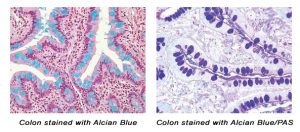
August Histology Special – Alcian Blue and PAS Staining
The combination of the Alcian blue and PAS stains can be used to distinguish neutral mucins from acid mucins. Mucins are a key component in lubrication, cell signaling, and forming chemical barriers. Using Alcian blue (pH 2.5), acid mucins will be stained a deep blue. The subsequent application of the PAS technique will stain the neutral mucins a bright magenta. Tissues or cells that contain both neutral and acidic mucins will be stained a dark blue or purple color.
The combined Alcian blue/PAS technique is the most sensitive and comprehensive method for detection of mucins, since all mucins should react regardless of their charge nature.
You’ll receive 30% off your Alcian Blue, PAS, or combination Alcian Blue/PAS staining in August when you mention this ad.
Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412) 624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
Have you noticed?
The McGowan Institute for Regenerative Medicine has a new website. Same location: https://mirm-pitt.net/. Same great content. New and improved format.
Take a look and let us know what you think!
Send your comments and suggestions to John Murphy or Rebecca Bauroth (baurothr@upmc.edu).
UPCOMING EVENT
9th Annual International Symposium on Regenerative Rehabilitation

The McGowan Institute for Regenerative Medicine invites you to join us for the 9th Annual International Symposium on Regenerative Rehabilitation, to be held October 27-29 in Austin, TX! Now in its 9th year, this symposium is the largest medical and scientific conference specific to Regenerative Rehabilitation in the world and brings together renowned experts in the fields of regenerative medicine and rehabilitation. This year’s featured speakers include, Theresa A. Jones, PhD (University of Texas at Austin), Karunesh Ganguly, MD, PhD (University of California, San Francisco), Conor Walsh, PhD (Harvard University), William R. Wagner, PhD (University of Pittsburgh), and José del R. Millán, PhD (University of Texas at Austin).
Join us in discovering integrated methods to enhance tissue healing and regeneration through mechanotransduction and applied biophysics, as well as how these approaches relate to the application of clinically available rehabilitation approaches. Cutting-Edge Research, Trainee Opportunities, Travel Awards, Diversity Grants, Networking and More!
View the agenda and register here.
SCIENTIFIC ADVANCES
Newsweek’s Top Plastic Surgeons in the USA

Newsweek magazine partnered with Statista Inc., the global market research and customer data firm, to find America’s Best Plastic Surgeons for 2022. The data was ranked into five categories: Breast Augmentation, Facelift, Liposuction, Eyelid Surgery, and Rhinoplasty. To determine the winners, Newsweek conducted a national survey among 2,000 medical professionals, asking them to recommend the best plastic surgeons in the business.
McGowan Institute for Regenerative Medicine faculty member Peter Rubin, MD (pictured), was named a top surgeon in four of the categories surveyed: Breast Augmentation (47 of 150), Facelift (86 of 150), Liposuction (20 of 150), and Eyelid Surgery (21 of 100).
Dr. Rubin is Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh. Dr. Rubin is well-recognized for his surgical skills and innovative solutions to complex aesthetic and reconstructive problems. He is Founder and Director of the Life After Weight Loss Surgical Body Contouring Program at the University of Pittsburgh. In addition to his active clinical program, Dr. Rubin directs a basic science research program in the biology of adipose-derived stem cells and serves as Co-Director of the Adipose Stem Cell Center at the University of Pittsburgh. He is the principal investigator in an NIH-funded line of research aimed at developing cell-based methods for clinical soft tissue reconstruction after cancer therapy. He directs a related line of research aimed at soft tissue reconstruction for injured military personnel as an investigator for the Department of Defense Armed Forces Institute for Regenerative Medicine (AFIRM). To facilitate the rapid translation of new technology, he founded the Center for Innovation in Restorative Medicine (CIRM) at the University of Pittsburgh.
His many scientific leadership positions include current Director, American Board of Plastic Surgery; Co-Chair of the American Society of Plastic Surgeons (ASPS) Task Force on Regenerative Medicine; Regulatory Chair for ASPS; Vice President of Finance for ASPS, past president of the International Society of Adipose Therapeutics and Science (IFATS), Board Chair of IFATS, and past Chairman of the Plastic Surgery Research Council. Dr. Rubin is the recipient of a Presidential Early Career Award for Scientists and Engineers (PECASE). The Presidential Award is the highest honor bestowed by the United States government on outstanding scientists and engineers early in their research careers. It is intended to recognize some of the finest scientists who show exceptional potential for leadership at the frontiers of scientific knowledge during the twenty-first century. He has served as editor for four textbooks, published over 180 peer reviewed articles, and presented over 500 invited lectures.
According to the ASPS’s most recent statistics, in 2020 Americans spent $16.7 billion on 15 million cosmetic medical procedures on their skin, including both surgery like liposuction and minimally invasive procedures like Botox injections and chemical peels. The most popular cosmetic surgery was rhinoplasty, followed in order by eyelid surgery, face lift, liposuction, and breast augmentation.
Congratulations, Dr. Rubin!
Concussion Incidence Increases the Risk of a Suicide Attempt in Children with a History of Depression

A child’s history of depression and concussion within the previous year, along with their race, ethnicity, and sex, can help predict the likelihood of a suicide attempt, according to a new study led by University of Pittsburgh School of Medicine researchers. McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD (pictured), Professor of Neurological Surgery and Director of the Neurotrauma Clinical Trials Center, Director of Neurotrauma and of the Scoliosis and Spinal Deformity Program at UPMC, and Clinical Director of the Brain Trauma Research Center, is a co-author of the study.
The study, published in JAMA Network Open, is one of the first to delve into the complex relationship between race, ethnicity, depression, and concussion in teenagers and pre-teens. The researchers gathered data from over 28,000 children in 9th through 12th grades who completed a national survey on their history of depression and whether they had a concussion caused by physical activity, which revealed eye-opening statistics about what groups were at risk for suicide attempts.
Among children who were depressed, concussion increased the risk for reporting a suicide attempt by 31%. If the child reported depression and concussion in the previous year, being Black, Hispanic, Latino, or multiracial enhanced risk for a suicide attempt by an additional 28%. Within that group, females had a 33% increased risk of a suicide attempt compared to males.
“Depression is a major predictor of a suicide attempt, which is why we wanted to look at children with and without depression. But our primary goal was to investigate if concussion would magnify risk for certain subgroups based on race, ethnicity, and biological sex,” said Shawn Eagle, PhD, lead author and research assistant professor in Pitt’s Department of Neurological Surgery.
They also found that for children without a history of depression, race and ethnicity played a greater role in predicting a suicide attempt than concussion incidence. Data showed that American Indian, Alaska Native, Black, Native Hawaiian, and Pacific Islander youth had an 89% increased risk of a suicide attempt compared to the rest of the survey respondents who did not report depression. In this group, concussion history did not increase the likelihood of a suicide attempt compared to race and ethnicity. However, concussion history did increase the risk for a suicide attempt within the remaining racial and ethnic groups by 29%.
Eagle points out that increased risk for suicide attempts with no reported depression history in certain racial and ethnic groups compared to others suggests how critical race and ethnicity can be to a child’s mental well-being in the United States.
This study included more than 28,000 survey participants, with 3,874 children reporting that they had suffered concussions in the last year. Overall, approximately 1,900 children said they had attempted suicide. Of those who had a suicide attempt, 80% reported experiencing depression within the year.
Data was collected from The Youth Risk Behavior Surveillance System (YRBSS), a Centers for Disease Control and Prevention-sponsored survey that monitors six categories of health-related behaviors that contribute to the leading causes of death and disability among youth and adults.
“Providers who work with children should conduct regular depression screenings and be mindful of certain subgroups that have increased risk for suicide attempts,” said Dr. Eagle. “And providers who work with children who may have had a recent concussion should conduct depression screenings more often.”
Prior studies have examined the association of concussions and suicide attempts in select minority groups as compared to white people, but this study is the first to compare ethnic and racial groups in a more detailed way, said co-author Anthony Kontos, PhD. He is director of research for the UPMC Sports Medicine Concussion Program and professor in the Department of Orthopaedic Surgery in Pitt’s School of Medicine.
“By looking more specifically at individual racial and ethnic groups, our findings provide more targeted data about each group’s risk factors, thereby improving our potential to develop more effective strategies to enhance patient care for these underserved groups,” he said.
The research team notes that the YRBSS data does not capture key variables and they cannot verify the information reported in the surveys. The surveys do not include details of when a child suffered a concussion or experienced depression, which could yield critical information about how these variables may directly lead to suicide attempts.
Dr. Eagle hopes future research will follow a large number of children over time to better understand how and why some groups are more vulnerable than others, and determine the timeline of concussion, depression, and suicide attempts.
Welcome: Drs. Chadi Hage and Daniel Forman

Chadi Hage, MD (pictured), renowned for his clinical expertise in treating patients diagnosed with common to complex lung conditions, has been appointed as medical director of the UPMC Lung Transplant program. He is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Dr. Hage’s research and clinical interests include fungal infections, lung transplant, extracorporeal membrane oxygenation (ECMO), and transplant critical care.
“With his arrival to UPMC, Dr. Hage will educate our team and offer care for patients from around the world with the most complex lung conditions,” said Pablo Sanchez, MD, chief, Division of Lung Transplant and Lung Failure and McGowan Institute affiliated faculty member. “Our lung specialists are national leaders, and our lung transplant program has become one of the leading centers in management and treatment of life-threatening lung diseases, and we welcome Dr. Hage to our team.”
Over the course of his 25-year career, Dr. Hage has treated patients with common and complex lung conditions, vastly researched various subjects pertaining to pulmonary medicine and worked to advance quality improvement and diversity, equity, and inclusion initiatives. He joins a multidisciplinary, nationally recognized team of physicians, investigators, and surgeons who carry on a legacy of clinical excellence and innovation that spans four decades.
Dr. Hage comes to UPMC from Indiana University Health, where he had served as associate professor of clinical medicine since 2014. He led the lung transplant research program and fungal research at Indiana University School of Medicine in addition to clinical trials that dealt with a patient’s immune system and its response to COVID-19.
He received his medical degree from Lebanese University School of Medicine and completed his residency and fellowship in infectious diseases and pulmonary-critical care medicine at Indiana University.
“I look forward to expanding the lung transplant program, developing multidisciplinary clinical programs, and helping our patients with lung complications lead full and healthy lives,” said Dr. Hage.
Dr. Hage is well published on endemic mycoses (histoplasmosis and blastomycosis), fungal infections of the immunocompromised patients, as well as outcomes of lung transplants.
UPMC’s Lung Transplantation Program is one of the most experienced centers in the world for lung and combined heart-lung transplantation. Since the program’s inception in 1982, the team has performed more than 2,350 lung and heart-lung transplants. The UPMC Lung Transplant Program works with the UPMC Comprehensive Lung Center to provide exceptional care for patients along the entire spectrum of lung diseases.
 The McGowan Institute for Regenerative Medicine also welcomes new affiliated faculty member Daniel Forman, MD (pictured).
The McGowan Institute for Regenerative Medicine also welcomes new affiliated faculty member Daniel Forman, MD (pictured).
Dr. Daniel Forman is a Professor of Medicine at the University of Pittsburgh in the Divisions of Cardiology and Geriatrics. He has faculty appointments at the University of Pittsburgh Medical Center (UPMC) and the VA Pittsburgh Health System (VAPHS). At UPMC, he is Chair of Geriatric Cardiology and Associate Director of Translational Research at the University of Pittsburgh’s Aging Institute. At the VAPHS, he is the Director of Translational Research of the Pittsburgh Geriatrics Research, Education, and Clinical Center (GRECC) as well as Director of Transitional Care with related leadership as Director of its Cardiac Rehabilitation and Gerofit programs.
Dr. Forman receives funding from the National Institutes of Health and the Veterans Health Affairs with research interests that center on physical function in older adults with cardiovascular disease. A National Institute on Aging grant focuses on nitrite supplementation to enhance skeletal muscle mitochondrial bioenergetics in older sedentary adults, and related bioenergetic and functional gains. Both National Institute on Aging and Veterans Health Administrative grants focus on novel implementation strategies of cardiac rehabilitation for older adults. In related studies, he is studying the benefit of inspiratory muscle training to improve skeletal muscle energetics and clinical outcomes in older adults with heart failure.
Dr. Forman has prominent leadership roles in the field of Geriatric Cardiology, with significant impact in the American College of Cardiology and the American Heart Association to shift research, guidelines, and practice management to incorporate scientific principles and outcome metrics pertinent to an older patient population.
Welcome!!
Dr. Jonathan Cheetham: Touching a Nerve

A passion for horses is foundational for the veterinarians at the Cornell Equine Hospital. It has led to groundbreaking procedures, state-of-the-art techniques, and an impressive wealth of knowledge that has saved and improved the quality of life of many animals. Up-and-coming experts are already making waves in the fields that Cornell legends pioneered, fostering an equine surgery frontier that is exciting both for the outlook of horses and the veterinarians who care for them.
McGowan Institute for Regenerative Medicine affiliated faculty member Jonathan Cheetham, VetMB, Diplomate ACVS, PhD (pictured), associate professor in the large animal surgery section, is making important forays into equine airway research, with a clinical interest in upper airway surgery and equine sports medicine. His research is applied to both human and veterinary patients.
After receiving his bachelor of veterinary medicine and master’s degrees from Cambridge University, Dr. Cheetham worked in first-opinion and referral equine practices in the United Kingdom for several years. He then came to Cornell for a residency in large animal surgery and completed his PhD in 2008 under the tutelage of equine airway pioneer Norm Ducharme, DVM, the James Law Professor of Surgery Emeritus. He joined the Department of Clinical Sciences as the Harry M. Zweig Research Scientist in 2012, followed by his appointment to associate professor in 2016.
“His research is really top-shelf,” says Dr. Ducharme. “The work he’s doing will have made an amazing difference even five, 10 years from now. It’s promising and exciting.”
Dr. Cheetham’s concern is with peripheral nerve repair — specifically, understanding the relationship between the immune response to nerve injury and recovery, and modulating that immune response to improve functional outcome after an injury, as well as restoring laryngeal function using regenerative medicine techniques combined with reinnervation.
“I think of my process as a sort of wheel,” says Dr. Cheetham. “The wheel can spin around, discovering at one point, modifying and translating at another and finally having the application from those findings spit out in unique ways.”
The connective thread that runs throughout Dr. Cheetham’s many projects — understanding the immune system’s initial response to nerve injury and leveraging it to improve patients’ outcomes — is proving successful in the equine larynx, as well as canine laryngeal and human peripheral nerve injuries, via collaborations with colleagues at Cornell’s Companion Animal Hospital, the Cornell University Hospital for Animals, and Weill Cornell Medicine, respectively.
This work on nerve injuries in the lab is approaching the exciting point of application in clinics. “We’re working on something that helps promote nerve growth after a nerve graft,” Dr. Cheetham says. “There are a few promising candidates that have seen success in mice, rats, and other species, so we’re looking forward to bringing that to the clinic.”
This project is building from Dr. Ducharme’s work. “This would be an addition to the amazing work he has done to encourage those nerves we graft onto the muscle to regrow more quickly,” says Dr. Cheetham.
Collaborating for clients and patients
From the operating room to work in the lab, Dr. Cheetham emphasizes that success relies on trusting team expertise. “Fellow surgeons will share their experience, the residents are great, and the overall environment is excellent,” he notes. “The technicians in the hospital have a sort of sixth sense about cases and have amazing relationships with clients. They’re a core part of the team. And from a research perspective — we have a rich environment that allows and encourages innovation. A strength is knowing that our work is supported by the college.”
Dr. Cheetham contributes to multiple services at the Cornell Equine Hospital, including the equine sports medicine and rehabilitation service; the soft tissue surgery service; the emergency and critical care service; the orthopedic service; and the regenerative therapies service. The Cornell Equine Hospital is a world leader in its field largely due to the expertise of the veterinarians and team members in each of these services and more.
Dr. Cheetham agrees it’s a team attitude that creates success. “We all feel free to exchange ideas and support each other.”
Experts Study Marine Mammals to Learn About Human Hearing

Many hearing loss patients have the same complaint: They have trouble following conversations in a noisy space. Carnegie Mellon University’s (CMU’s) Barbara Shinn-Cunningham, PhD, has spent her career conducting research to better understand this problem and how it affects people at cocktail parties, coffee shops, and grocery stores.
Now, along with a team of researchers from six universities, Dr. Shinn-Cunningham, the director of CMU’s Neuroscience Institute (NI) and the George A. and Helen Dunham Cowan Professor of Auditory Neuroscience, is looking for answers in an unexpected place. The researchers will conduct noninvasive experiments on free-swimming dolphins and sea lions.
To develop those methods, the research team turned to McGowan Institute for Regenerative Medicine affiliated faculty member Jana Kainerstorfer, PhD (pictured), a CMU associate professor of biomedical engineering and an expert in multimodal imaging techniques. She and her team are currently investigating ways to apply the technologies, which are commonly used in humans, to sea lions.
“By using inherently noninvasive methods, we are able to learn something about the animals’ behavior and brain processing without interfering with their behavior,” Dr. Kainerstorfer said. “It’s incredible to have people from such diverse backgrounds come together and work on a common problem.”
Dolphins and sea lions hear differently than humans, but the way their brains make sense of sound could help Dr. Shinn-Cunningham and her team better understand the cognitive and neural mechanisms of sound processing. The researchers have received a Multidisciplinary University Research Initiative grant from the Department of Defense to investigate how animals understand and respond appropriately to the cacophony reaching their ears.
The team will conduct several different experiments.
Working with a world expert on dolphin behavior, they will train dolphins to identify targets, shapes such as a spheroid or a cross, and then ask the dolphin to identify the target from among similar, distracting shapes using their special auditory sense: echolocation. By engineering the distractor shapes to be more or less like the target, the experimenters will determine what sound features the dolphins use to “see” with sound.
In a different series of studies, the team will explore whether dolphins, like humans, learn to anticipate repeating patterns in sound, a critical step in making sense of complicated sounds in noisy settings. In this work, they will use electroencephalography (a technology used routinely with humans in laboratories and clinics) to measure neural responses from the dolphins to see if the brain shows a surprise signature when the ongoing sound pattern changes.
Later, the scientists will use sea lions to investigate whether, as in humans, different brain networks calculate the location of a sound versus the meaning of a sound. They will train the animals to respond to where a sound comes from (left or right) or what the sound is (e.g., the call of another sea lion or the sound of the trainer’s voice). Using near-infrared spectroscopy, the same technology often used in doctor’s offices on human fingertips to determine oxygen levels, the researchers will examine whether the brain regions engaged when listening to the same sounds change depending on what the sea lion is calculating.
Dr. Shinn-Cunningham said that the research could point to new approaches to developing hearing aids and other assistive listening devices, along with other benefits.
“Developing noninvasive ways of monitoring brain function in these marine mammals, including when in the wild, can give us insight into how these animals navigate in and make sense of their undersea world, which is important to conservation efforts,” she said. “Additionally, learning how the brains of our marine cousins process complex acoustic scenes, and how that is similar or different to auditory processing in humans, can give us a deeper understanding of human hearing. I appreciate that we have access to phenomenal, accredited research facilities for this work.”
It Takes a Heart to Make a Better Lung
Interactions between the developing heart and lungs are essential for proper growth and maturation; however, much is still unknown about the co-development of these critical organs. To provide fresh insight, a team of collaborators from Carnegie Mellon University (CMU), Boston University, and the University of Pittsburgh recently presented the first laboratory model for human heart and lung co-development to help researchers discover new strategies to probe the underlying mechanisms of cardio-pulmonary interactions.
Organs begin to take shape during the first few months of life as the baby grows as an embryo inside the womb. During this embryogenesis, different layers of stem cells are activated to turn into specific types of cells, for example, a heart or lung cell. The heart and lungs develop from two distinct germ layers within the embryo, the mesoderm and endoderm, which must communicate with each other for the organs to form correctly.
“My scientific training is rooted in developmental biology,” explained Xi “Charlie” Ren, PhD, assistant professor of biomedical engineering at CMU and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. “I was curious about how we could borrow learnings from embryogenesis to achieve a deeper understanding of when something goes wrong with our critical organs, like what we see in congenital diseases. Animal models offer some answers, but there are key differences that prevent us from really translating data for human understanding. We set out to generate a model that could be directly applied to human health.”
In work published in eLife, the group developed a human model of heart and lung co-development during embryogenesis using lab-grown human induced pluripotent stem cells (hiPSCs). The hiPSCs were treated with chemical signals, causing them to form different germ layers that developed into early forms of heart and lung cells. Then, the cells were transferred into a tailored growing condition, where they arranged into three-dimensional structures termed microtissues.
Interestingly, the research revealed that lung cells matured faster when grown in microtissues accompanied with developing heart cells, when compared to developing lung cells alone.
“Traditionally in a lab setting, we’ve developed organs separately, to curb technical barriers related to balancing the lineage of two organs and a recipe that was needed to support both of them,” noted Dr. Ren. “However, through this novel co-development model, we found that the lung needs the heart. We’ve been able to show that when the heart develops with a lung and provides beneficial factors, the lung will mature better and faster. Currently we are studying the underlying molecular mechanisms of this intriguing phenomenon.”
“This is a truly exciting model of heart and lung co-development that provides important insight not only into embryogenesis, but also presents new strategies for bioengineering replacement heart and lung tissue for therapeutic applications,” added Adam Feinberg, PhD, CMU professor of biomedical engineering and materials science and engineering, a co-author on the study, and an affiliated faculty member of the McGowan Institute.
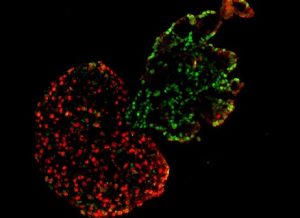
The model also demonstrated that co-developing heart and lung tissues automatically separate from each other during later stages, establishing an accurate boundary between the two neighboring organs. McGowan Institute faculty member Donna Stolz, PhD, associate director of the Center for Biologic Imaging, University of Pittsburgh School of Medicine, and an associate professor in the Departments of Cell Biology and Pathology at the University of Pittsburgh, is also a co-author of the study.
“Through this work, we named a new term: cardiopulmonary tissue segregation,” said Dr. Ren. “We observed that the cells seem to have some sort of intelligence to know their ‘closer’ friend; the lung cells automatically came together without leaving one cell behind, and the heart cells did the same. As they developed, they moved away from each other and separated. It’s almost magic.”
As stem cell engineering continues to rapidly develop, investigating the co-development of organs will continue to be a topic of interest. The group’s dynamic model and work related to studying multi-organ groups is ongoing.
Illustration: Segregation of heart and lung microtissues. The red represents heart microtissues, while the green represents lung microtissues. Carnegie Mellon University College of Engineering.
Dr. Tetsuro Sakai Selected for ILTS Special Interest Groups Steering Committee

Congratulations to McGowan Institute for Regenerative Medicine affiliated faculty member Tetsuro Sakai, MD, PhD (pictured), who was selected as a member of the Steering Committee of the Live Donor Liver Transplantation (LDLT) Special Interest Groups of the International Liver Transplant Society (ILTS) for a three-year term. Dr. Sakai is the only anesthesiology representative among the committee’s nine international LDLT experts.
Dr. Sakai is a Professor of the Department of Anesthesiology and Perioperative Medicine and a Professor of the Clinical Translational Science Institute, University of Pittsburgh School of Medicine. He is also a board-certified staff anesthesiologist (Hepatic and Intestinal Transplantation Anesthesia) in the UPMC Department of Anesthesiology and Perioperative Medicine. Dr. Sakai’s current research interests include education in anesthesiology (resident research education); liver transplantation anesthesiology; and quality improvement in anesthesiology.
The ILTS is a growing organization of approximately 1400 international members belonging to a multidisciplinary cadre of specialists and healthcare providers who have joined to promote the focus of ILTS – the advancement of the science and practice of liver transplantation by sharing of clinical and educational experiences and research at an international level.
The primary goal of the LDLT Special Interest Group (SIG) of the ILTS is education, benchmarking, and best practice establishment in LDLT across the fraternity and across specialties involved in LDLT. The LDLT SIG will lead advances in the field and foster international collaborative scientific efforts.
Congratulations, Dr. Sakai!
Self-Assembled, Interlocked Threads
The spiral is pervasive throughout the universe — from the smallest DNA molecule to ferns and sunflowers, and from fingerprints to galaxies themselves. In science the ubiquity of this structure is associated with parsimony — that things will organize themselves in the simplest or most economical way.
Researchers from the University of Pittsburgh and Princeton University unexpectedly discovered that this principle also applies to some non-biological systems that convert chemical energy into mechanical action — allowing two-dimensional polymer sheets to rise and rotate in spiral helices without the application of external power.
This self-assembly into coherent three-dimensional structures represents the group’s latest contribution in the field of soft robotics and chemo-mechanical systems.
The research was published in Proceedings of the National Academy of Sciences (PNAS) Nexus. Lead author is Raj Kumar Manna, PhD, with Oleg E. Shklyaev, PhD, post-doctoral associates with McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical and Petroleum Engineering and the John A. Swanson Chair of Engineering in Pitt’s Swanson School of Engineering. Contributing author is Howard A. Stone, PhD, the Donald R. Dixon ’69 and Elizabeth W. Dixon Professor of Mechanical and Aerospace Engineering at Princeton.
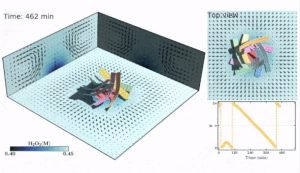
“Through computational modeling, we placed passive, uncoated polymer sheets around a circular, catalytic patch within a fluid-filled chamber. We added hydrogen peroxide to initiate a catalytic reaction, which then generated fluid flow. While one sheet alone did not spin in the solution, multiple sheets autonomously self-assembled into a tower-like structure,” Dr. Manna explained. “Then, as the tower experienced an instability, the sheets spontaneously formed an interweaving structure that rotates in the fluid.”
As Dr. Balazs pointed out, “The whole thing resembles a thread of twisted yarn being formed by a rotating spindle, which was used to make fibers for weaving. Except, there is no spindle; the system naturally forms the intertwined, rotating structure.”
Flow affects the sheet which affects the flow
Further analyzing the results, Dr. Manna found that tiny random fluctuations in the local concentration of the reactant create sufficient torque for the four sheets suspended in the fluid to be dragged upward, intertwine, and rotate. This interlinking phenomena occurs innately when the reactants and products have different volumes, which creates a density gradient in the presence of gravity.
“It was a surprise to see these simple 2D sheets form a complex spiral just by “sprinkling” reactant — such as a teaspoon of glucose — into the chamber,” Dr. Balazs said. “Doubling the number of sheets to eight increased the complexity of the spiral. We wondered if this was the tip of the iceberg — if we pressed further, could we develop the parameters to achieve various dynamic movements, which would be critical in programming soft robotics.”
Dr. Shklyaev added, “Devices are typically three-dimensional and not two, so by creating the design rules, we could increase the complexity of the rotating structures formed by the sheets. Anna provided the inspiration with the painting La Danse by Henri Matisse and asking if we could replicate the dancers’ poses.”
Drs. Manna and Shklyaev then added extensions to the four sheets, making t-shaped structures resembling outstretched arms. According to Dr. Shklyaev, changing the shape of the sheets enabled them to “tune” the sheets’ movement to resemble a coordinated circle dance.
According to Dr. Balazs, the researchers can quantify how the interlinked sheets should be designed and arranged, thereby enabling others to further develop more robust, scalable systems. Additionally, varying the shape of the container holding the fluid — which was rectangular in their modeling — provides another handle for tailoring the systems’ dynamic response.
“When you have a release of chemical energy into a fluid, it is then transduced into mechanical energy, which can perform specific actions. And although the process dissipates energy, simply adding another small amount of reactant reactivates it,” she said. “The next stage in our study is to program passive and active sheets to form other interwoven, three-dimensional structures, now that we know how to control their responses.”
Illustration: Dynamic self-assembly and self-rotation of eight passive sheets in a fluid-filled chamber. (Raj Kumar Manna)
Recruiting Patients – Clinical Trial – Volumetric Muscle Loss
Previously reported are the results of a study conducted by researchers at the University of Pittsburgh School of Medicine and the McGowan Institute for Regenerative Medicine that showed significant improvement in strength and range of motion, as well as evidence for skeletal muscle regeneration, in 13 patients who were surgically implanted with bioscaffolds derived from pig tissue to treat muscle injuries. The patients had failed to respond to conventional treatment before use of the extracellular matrix (ECM). The findings were published online in npj Regenerative Medicine.
In the prior, completed human subject clinical trial (NCT01292876, PRO10010500) the researchers evaluated a regenerative medicine approach using ECM for volumetric muscle loss (VML) treatment. ECM scaffolds were implanted and combined with aggressive and early physical therapy in 13 subjects, then followed for 24-28 weeks after implantation. Histomorphological assessments collected from core needle biopsies identified formation of new, vascularized, innervated islands of skeletal muscle within the implantation site. Subjects demonstrated increased force production in physical therapy evaluations and improved functional task performance when compared with pre-operative performance. By 6 months after ECM implantation, subjects had a 37.3% improvement in strength and 27.1% improvement in range-of-motion tasks. Additionally, changes in nerve conduction study (NCS) and electromyography (EMG) before and after ECM implantation were measured. 63% of study participants experienced improvements in NCS or EMG within the scaffold remodeling site, indicating clinical improvement in muscle strength. The promising functional and regenerative results from this early study encourages evidence of ECM bioscaffolding as a viable treatment to VML.
The research and clinical teams are now recruiting patients for a second DoD-funded clinical trial to further assess the effectiveness of ECM implants to restore muscle function in patients who have lost muscle due to trauma. [ClinicalTrials.gov Identifier: NCT04051242.]
This study proposes to use XenMatrix™ AB Surgical Graft which has 510(k) approval intended for implantation to reinforce soft tissue where weakness exists and for surgical repair of damaged or ruptured soft tissue, including abdominal plastic and reconstructive surgery; muscle flap reinforcement; hernia repair including abdominal, inguinal, femoral, diaphragmatic, scrotal, umbilical, and incisional hernias. The graft has an antibiotic coating. This coating has been shown in preclinical in vitro and in vivo testing to reduce or inhibit microbial colonization on the device. The claim of reduction of bacterial colonization of the device has not yet been established with human clinical data, nor has a clinical impact associated with this claim been demonstrated and will need further investigation. This trial proposes to test the applicability and utility of XenMatrix™ AB Surgical Graft in the restoration of function in the setting of volumetric muscle loss after trauma. Ten subjects will be enrolled for participation in the study. Prior to Graft implantation, subjects will receive a pre-operative course of physical therapy for a maximum time period of 16 weeks. A physical therapist will confirm that functional plateau is reached prior to implantation of the Graft. Following Graft implantation, radiographic, functional, and electrophysotherapy outcomes will be measured at various time points up to 24-28 weeks post-operatively. A CT scan or MRI will be collected at screening and pre-operative visits to evaluate tissue volume, then again at post-operative Visit 1 and Visit 6. Physical therapy training will be performed as a research procedure following Graft implantation for a maximum of 30 weeks. Additionally, physical therapy evaluations will be conducted at screening, pre-op visit 1, post-op, and at post-op Visits 3, and 4. A small core needle biopsy 1-5 grams will be collected at three time points to conduct histomorphological assessment of the tissue prior to Graft implantation (Operative visit, Visit 2, and at Visit 4).
To explore eligibility and possible enrollment in the new clinical trial please inquire at 412-641 8676, or woundresearch@pitt.edu. For more background on this study, listen to the podcast with McGowan Institute deputy director Stephen Badylak, DVM, PhD, MD, here.
Pitt’s RNEL and Blackrock Neurotech to Expand Reach of Brain-Computer Interface Trials with In-Home System

Brain-computer interface (BCI) trials may soon be more accessible to a greater population of candidates living with paralysis, per a new partnership agreement between the University of Pittsburgh’s Rehab Neural Engineering Labs (Pitt RNEL) and Blackrock Neurotech, a leader in implantable brain technology. The agreement sets the stage for Pitt RNEL to scale future BCI studies, enabling more people with remote BCI systems to participate in study sessions from their homes. Pitt RNEL is also creating a process to expand and improve participant recruitment, both remote and on-site, ultimately enabling larger studies with greater numbers of volunteers.
Since 2004, implantable BCIs have offered people with spinal cord injuries and other neurological disorders the potential to restore abilities. These BCI systems—comprised of miniaturized electronics, hardware, and machine learning software—decode and translate brain signals into digital commands, providing people with paralysis the ability to control external devices such as computer cursors or robotic arms.
Pitt was among the first research centers to implant Blackrock’s BCI in human participants and has since conducted pioneering trials on a range of BCI applications, including complex control of robotic limbs and restoring sensory feedback using electrical stimulation of the brain. However, participation in Pitt’s clinical trials has, up to now, largely been limited to people living within driving distance of the institution.
“Typical experiments require participants to come into our lab facilities on a regular basis, which is a successful model that will continue to be essential in pushing BCI science forward for years to come,” said McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD (pictured), professor of physical medicine and rehabilitation and investigator in the Rehab Neural Engineering Labs at Pitt. “However, home testing has become an important supplement to lab testing whereby we enlist study staff to attend sessions and transport parts of the lab equipment to the participant’s home to conduct testing. With this new initiative, we will develop a miniaturized and streamlined BCI system designed to work in people’s homes, while replicating the potential for complex lab-based experiments.”
Under this newly funded collaboration, BCI sessions using this new system will occur at the homes of people participating in the study, demonstrating that BCI research can extend outside of the greater Pittsburgh area. By reducing the challenge of study-related travel, researchers can test a broader population of participants and collect more diverse safety and efficacy data. Importantly, these in-home BCI trials are distinct from other at-home trials Pitt has conducted, where portable systems have been used independently by participants for limited testing and recreational purposes.
The Pitt RNEL group has previously shown that a portable BCI system could be successfully used at home to perform a variety of tasks by imagining the movement of a mouse cursor on the system’s integrated monitor, ranging from playing computer games and typing sentences to creating digital art.
“I first started using the portable system on my own in the summer of 2020, when COVID shut down testing at the lab,” said study participant Nathan Copeland. “I think in-home devices are the next essential step for getting BCI technology into the brains of the people that could have their lives improved by it. Whether it’s to draw cute cats, play video games, or as a primary tool for communication, the capability of using the system in the real world beyond the confines of a lab building will start changing lives and how people think about BCI.”
The expanded trials and compact system are an important step in Blackrock’s efforts to make the first BCI platform commercially available to people with paralysis. The technology, which has been chronically implanted in humans since 2004, has until now only been available to patients through research studies – but Blackrock believes it is ready for real-world use.
“At a neuroscience conference a few years ago, three participants in clinical trials of Blackrock devices sat on a panel and expressed a consensus that our technology addressed immediate needs in their lives,” said Marcus Gerhardt, CEO and co-founder of Blackrock. “Through this expansion of our partnership with Pitt, we will be able to connect with and learn from larger patient populations, with the guiding vision of making the technology available to as many patients as possible.”
More information on the remote trials and recruitment efforts at the University of Pittsburgh can be found here.
Transplantation Science and the Department of Defense: A Unique Partnership

The Department of Defense isn’t what most people would associate with the field of transplantation science, but for McGowan Institute for Regenerative Medicine affiliated faculty member Hēth Turnquist, PhD (pictured), associate professor in the Department of Surgery and the Thomas E. Starzl Transplantation Institute, they are a major partner for his work. Since 2011, Dr. Turnquist has led a transplant immunology-focused research program that is working to understand how immune cells respond to tissue damage. Their goal is to now harness their new knowledge on the mechanisms the immune system uses to regulate the responses of other immune cells and complete tissue repair after injury for the benefit of transplant recipients.
One subset of immune cells that has been of particular interest to Dr. Turnquist’s team are regulatory T cells, also called Tregs. Tregs have long been known to have an important role in regulating or suppressing potentially detrimental immune responses and help prevent autoimmune disease. Learning more about how to augment natural immunosuppression by Tregs to limit recipient immune response to grafts could be a key to reducing or eliminating chemical immunosuppressive drugs, which come with many difficult side effects.
Among his lab’s many discoveries, Dr. Turnquist’s studies have identified a unique and rare subset of Tregs that are suppressive, but also support tissue repair. These reparative Tregs (RepTregs) respond to signals released during injury to direct other cells to begin the tissue repair process and stimulate local tissue cell survival. The lab hopes that new findings such as these, combined with advances in surgery methods and bioengineering of drug delivery systems, will become the pathway towards inducing transplantation tolerance and reducing long-term tissue injuries leading to eventual loss of graft function.
Just where does the Department of Defense fit into this? According to Dr. Turnquist, there are more than 1600 U.S. military amputees as a result of missions during Operations Iraqi Freedom, Enduring Freedom, and New Dawn alone, with over one third being dual amputees. There are also an estimated 2 million Americans living with limb loss. A procedure called vascular composite allograft transplantation (VCA) can help by offering a transplantation of a functional unit of multiple tissues such as skin, muscle, tendon, nerve, and bone. Example VCA transplants include hands and face transplants. However, the widespread use of VCA is limited by the toxicity and complications of the high-dose, multi-drug immunosuppression necessary to prevent graft rejection by the recipient’s immune system. Even when used at toxic doses these drugs fail to prevent transplant fibrosis that limits transplant function. Through Dr. Turnquist’s work to better understand RepTregs, he hopes to set the groundwork for new therapies with the potential to lead to clinical trials in the reduction of immunosuppression needed for VCA recipients, while also improving long-term graft functions.
Though human clinical trials are potentially 5-10 years away, if effective, these studies could potentially launch a new avenue of investigation and novel drugs that can harness potent repair pathways in damaged graft tissues. This would not only affect the quality of life for wounded military veterans, but many other patients worldwide who suffer from limb loss. Dr. Turnquist and his lab are on the frontlines of the search for new treatments to help those who were on the frontlines once themselves.
AWARDS AND RECOGNITION
Dr. Erin Sullivan Achieves Faculty Promotion

McGowan Institute for Regenerative Medicine affiliated faculty member Erin Sullivan, MD, D.ABA, FASA, is now a Professor of Anesthesiology within the Department of Anesthesiology and Perioperative Medicine at the University of Pittsburgh School of Medicine.
Dr. Sullivan has earned many accolades and achievements in her field throughout her career. Just a few key highlights are her leadership as Chief of the Cardiothoracic Anesthesiology Division over the past 15 years and now being promoted to the role of the Associate Vice Chair in the department. She was also the Program Director of the Adult Cardiothoracic Fellowship from 2002 through 2020.
Dr. Sullivan has been appointed to several committees of professional societies like the Society of Cardiovascular Anesthesiologists (SCA) and American Society of Anesthesiologists (ASA), and she serves on the editorial board of the Journal of Cardiothoracic and Vascular Anesthesia and as an Oral Board Examiner and Senior Editor/Question Writer for the American Board of Anesthesiology (ABA) Applied Examination. Previously, Dr. Sullivan served as a question writer for the ASA Self-Education and Evaluation Program and the ABA-ASA Joint Council on In-Training Examinations.
Dr. Sullivan has authored or co-authored nearly 100 publications on her work, including an impressive 18 book chapters published in medical textbooks. Importantly, she holds prominent leadership positions in the ASA – Section Chair on Subspecialties for the ASA Division of Scientific Affairs and District IX Director for Pennsylvania on the ASA Board of Directors.
Congratulations, Dr. Sullivan!
Dr. Zachary Freyberg Named Psychiatry Research Pathway Associate Director

The specialized Psychiatry Research Pathway (PRP) enables Department of Psychiatry residents to simultaneously receive clinical training and pursue their passion for research through mentorship, conducting research projects, and receiving training that facilitates their career development into highly successful investigators. McGowan Institute for Regenerative Medicine affiliated faculty member Zachary Freyberg, MD, PhD, Assistant Professor of Psychiatry and Cell Biology will serve as PRP Associate Director.
The overall long-term goal of Dr. Freyberg’s research is to better understand human disorders of dopaminergic neurotransmission including addiction, schizophrenia, and Parkinson’s disease. In order to do so, his laboratory has begun dissecting presynaptic dopaminergic neurotransmission at the synaptic vesicle level using a combination of complementary approaches including genetics, pharmacology, and both light and electron microscopy. Consequently, the lab’s current research projects include Mechanisms of Dopamine Synaptic Vesicle Loading and Release in Health and Neurodegenerative Diseases; Dopamine’s Roles Outside the Central Nervous System in Regulation of Insulin Release and Antipsychotic Drug Action; In Situ Cryo-Electron Microscopy and Super-Resolution Imaging Approaches to Vesicle Trafficking and Synaptic Plasticity; and In Situ Cryo-Electron Tomography Approaches to Super-Resolution Precision Medicine for Mitochondrial Diseases.
Congratulations, Dr. Freyberg
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#235 –– Dr. Michael Boninger discusses his research in the development and application of assistive, rehabilitative, and regenerative technologies.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author:
Arun Sharma; Rachel A. Clemens; Orquidea Garcia; D. Lansing Taylor; Nicole L. Wagner; Kelly A. Shepard; Anjali Gupta; Siobhan Malany; Alan J. Grodzinsky. Mary Kearns-Jonker, Devin B. Mair; Deok-Ho Kim; Michael S. Roberts; Jeanne F. Loring; Jianying Hu; Lara E. Warren; Sven Eenmaa; Joe Bozada; Eric Paljug; Mark Roth; Donald P. Taylor; Gary Rodrigue; Patrick Cantini; Amelia W. Smith; Marc A. Giulianotti; William R. Wagner
Title: Biomanufacturing in low Earth orbit for regenerative medicine
Summary: Research in low Earth orbit (LEO) has become more accessible. The 2020 Biomanufacturing in Space Symposium reviewed space-based regenerative medicine research and discussed leveraging LEO to advance biomanufacturing for regenerative medicine applications. The symposium identified areas where financial investments could stimulate advancements overcoming technical barriers. Opportunities in disease modeling, stem-cell-derived products, and biofabrication were highlighted. The symposium will initiate a roadmap to a sustainable market for regenerative medicine biomanufacturing in space. This perspective summarizes the 2020 Biomanufacturing in Space Symposium, highlights key biomanufacturing opportunities in LEO, and lays the framework for a roadmap to regenerative medicine biomanufacturing in space.
Source: Stem Cell Reports: Perspective. 2022 Jan 11;17(1):1-13. doi: 10.1016/j.stemcr.2021.12.001. Epub 2021 Dec 30.
GRANT OF THE MONTH
PI: Stephen Badylak
Title: REPAIR: Regenerative Electronic Platform through Advanced Intelligent Regulation
Description: We will engineer the Regenerative Electronic Platform through Advanced Intelligent Regulation (REPAIR) Patch, which will dramatically improve the speed and functional outcome of wound healing. The technologic basis of our approach is the use of a novel array of sensors and actuators, designed and controlled through the use of computational models and embedded within an inductive cytocompatible extracellular matrix (ECM) hydrogel. The REPAIR Patch will be developed and tested in a dog model of volumetric muscle loss (VML). The REPAIR platform, which is flexible with regard to geometry, time of application, and arrangement of its various modalities will decrease by 50% the time to functional healing of VML wounds by targeting two key rate limiting steps in the default wound healing process: the immune phenotype of the wound environment and neurogenesis. The REPAIR technology fundamentally changes the current empirical, reductionist approach to wound healing.
The REPAIR approach will be driven by tissue-realistic, dynamic, agent-based computational models that are spatiotemporally accurate facsimiles of key biological processes involved in the in vivo experimental model. Initiating from in silico studies to define novel control points, and combined with in vitro studies to demonstrate feasibility through an iterative process, we will create this control strategy by utilizing; 1) experimental data (at the mRNA and protein levels) obtained from the canine studies; 2) dynamic, data-driven models that will recapitulate principle drivers and define central sense/actuate nodes; 3) modifications of the agent-based models to account for these novel sense/actuate nodes and predict experimental outcomes; 4) modification of the REPAIR Patch to carry out the model-defined sense/actuate process and validate predictions from the agent-based models; 5) the use of the agent-based models as proxy models to train an artificial intelligence (AI) controller given the sense/actuate capabilities of the REPAIR Patch, and 6) in vivo testing of the modified REPAIR Patch under closed loop control of the AI controller. Importantly, the REPAIR Patch will be applied, removed, and re-applied repeatedly to rebuild functional tissue in layers. If successful, the REPAIR platform will improve the quality of life of severely injured warfighters and civilians alike.
Source: Defense Advanced Research Projects Agency
Term: May 1, 2022 – June 30, 2023
Amount: $257,140
