January 2019 | VOL. 18, NO. 01| www.McGowan.pitt.edu
A Single Injection May Mean a Permanent Cure

Julie Grant, reporting for Pittsburgh’s local CBS affiliate, KDKA, recently visited with McGowan Institute for Regenerative Medicine affiliated faculty member George Gittes, MD, Director of the Richard King Mellon Foundation Institute for Pediatric Research and Co-Scientific Director at UPMC Children’s Hospital of Pittsburgh of UPMC. She learned that reversing autoimmune type 1 diabetes without immunosuppression has proven to be extremely difficult, but Dr. Gittes and researchers at Children’s Hospital and the University of Pittsburgh School of Medicine have achieved that outcome in pre-clinical trials with an engineered, safe virus that does gene therapy.
“We infuse the viruses into the pancreatic tissue. It permeates through the pancreas, but it finds the cells that are the ones that have the capability to turn into insulin cells and it makes that happen,” said Dr. Gittes.
The researchers have pioneered a novel procedure — an infusion process using an endoscope that delivers the virus directly to the pancreas, so other cells in the body are not affected.
“In a human, we could go through the mouth, down the stomach, to where the pancreas opening is, infuse the virus back up into the pancreas,” said Dr. Gittes.
One infusion could mean long-term results that would allow a diabetic patient to regulate their own blood sugar, replacing the need for insulin injections.
“The idea that you could do a single injection and see a permanent change is very exciting,” said Dr. Gittes.
A clinical trial for gene therapy to treat diabetes has never been done. Dr. Gittes’ team appears to be closer than ever, already having success in pre-clinical trials.
“Once we get a consistent result in the lab …, we will then go to the FDA and present them with the trial we want to do in diabetic patients,” said Dr. Gittes.
According to Dr. Gittes, they are pretty close to being ready for a clinical trial. This means closer, than perhaps ever before, to a cure.
In type 1 diabetes, the body mistakenly recognizes the insulin-producing ‘beta’ cells in the body as foreign and kills them, resulting in high blood sugar levels. Patients require lifelong insulin therapy either through injections or an insulin pump. The team’s current work however, represents a major advance in efforts to develop a long-term therapeutic approach by stimulating the body’s own pancreatic cells to produce insulin.
RESOURCES AT THE MCGOWAN INSTITUTE
February Histology Special
The McGowan Institute Histology Core wants to be your Valentine this month with big savings that will warm your heart. Bring your cardiac tissue to the McGowan Histology Core and receive 25% off your entire order in the month of February, when you mention this ad. From our hearts to yours, you won’t beat these prices, or our rapid turnaround times. Race in today for top quality results!
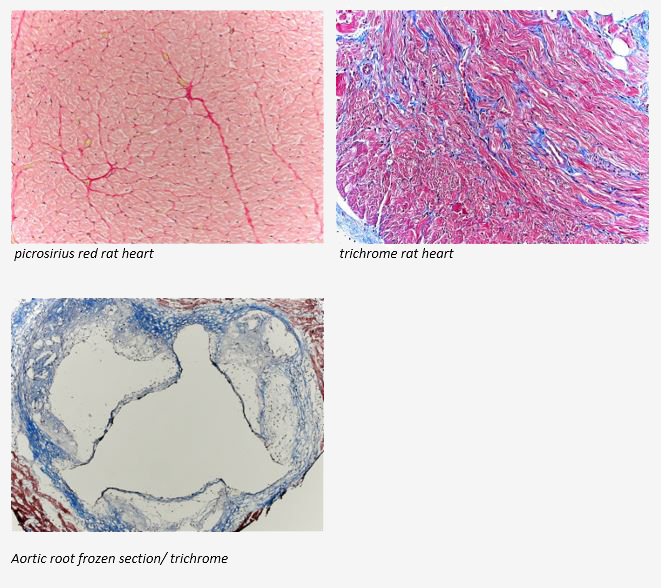
Contact the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology work with the quickest turn-around time.
Flow Cytometry Technical Applications Seminar
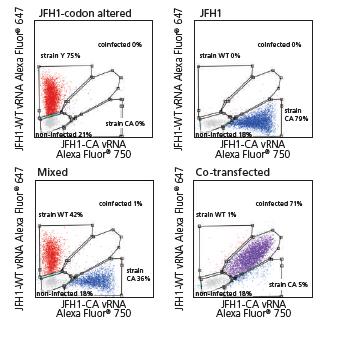
Noah Alberts-Grill, PhD will be presenting, “Protein and RNA Detection at the Single-Cell Level: PrimeFlowRNA Assay” on Tuesday, February 19th, at 1:00 pm – 3:00 pm. Dr. Alberts-Grill is the Field Application Scientist at Thermo Fisher Scientific. In this seminar, we will discuss PrimeFlowRNA, a novel bDNAin situhybridization assay to quantify protein and RNA expression simultaneously in heterogeneous cell populations. Topics will include:
- Introduction to bDNAtechnology
- Experimental design and work flow
- Building multicolor staining panels for protein and RNA
- Recent high-impact publications utilizing PrimeFlow(virology, immuno-oncology, basic immunology, etc.)
Please RSVP to Lynda Guzik, McGowan Flow Facility at guzilj@upmc.edu. Refreshment will be provided.
Photo: JFH1 HCV and assessed by PrimeFlowusing virus-specific probe sets. Cells were also either co-infected or mixed post-single infections. Data courtesy of van Buuren et al, Stanford University School of Medicine.
SCIENTIFIC ADVANCES
BYU Radio Hosts Interview with Dr. Stephen Badylak

Lizards can regrow an entire tail and salamanders can regrow a leg. Unfortunately, our human bodies mainly just close wounds and make scar tissue. But just imagine the possibilities if we could grow a new limb after an amputation or a new organ, rather than needing a transplant? That is the focus of a recent interview by Julie Rose, BYU Radio, with McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, professor in the Department of Surgery and director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute.
Ms. Rose and Dr. Badylak’s conversation touches on regenerative vs nonregenerative species and the similarities and differences of specific genome pathways which make regeneration possible. They also discuss the several regenerative tissues/organs in humans, namely the liver, the outer layer of skin, the inner intestinal layer, and the bone marrow. Dr. Badylak explains that all our human cells have the same DNA since conception, but after the first trimester of development they lose the signals to regrow tissues because that’s when the regenerative genes are turned off.
The use of mammalian extracellular matrix (ECM) or its derivatives as an inductive template for constructive remodeling of tissue is a common theme of most research activities in Dr. Badylak’s laboratory. His pioneering work has revolved around the structure and composition of naturally occurring ECM, and the signaling provided by this matrix to host cells toward functional tissue reconstruction. Dr. Badylak talks about his lab’s recent volumetric muscle loss study in which 13 military personnel participated. The results of the study showed 35-38% of missing muscle tissue was regrown with the use of ECM. More importantly, these results had a positive impact on the patients’ quality of life.
Dr. Badylak also explained about what his lab is doing in the area of whole organ engineering, specifically with the liver. The long-term goal of this work is to establish the decellularization, recellularization with autologous cells (from the patient, thus avoiding the need for subsequent immunosuppression), and transplantation criteria necessary to produce functional bioengineered organs for clinical translation.
A winner of multiple Edward R. Murrow Awards, Julie Rose is a seasoned broadcast journalist and interviewer. Prior to joining BYU Radio, Ms. Rose worked as a reporter and produced spots and feature news stories for NPR’s Morning Edition and All Things Considered.
Pennsylvania Pediatric Medical Device Consortium

The Pennsylvania Pediatric Medical Device Consortium (PPDC) has announced a partnership with two programs at the University of Pittsburgh. Formerly the Philadelphia Pediatric Medical Device Consortium, the PPDC’s new name reflects its statewide reach. This expansion comes on the heels of a five-year, $6 million grant renewal from the Consortium’s sponsor, the U.S. Food and Drug Administration.
Based at Children’s Hospital of Philadelphia (CHOP), the PPDC’s mission is to support the development of promising medical devices that address unmet clinical needs in children. It has assisted more than 60 innovative projects and over the past five years, the PPDC has awarded 16 seed grants of up to $50,000 each to companies in the Philadelphia region and beyond. The PPDC conducts a competitive process to select its award recipients.
The Consortium’s new cross-state partners are the McGowan Institute for Regenerative Medicine and sciVelo, both based at the University of Pittsburgh. Together, these programs focus on developing and commercializing biomedical technology.
“Our new partnerships with these outstanding programs build on our existing success in collaborating with Drexel University and the University of Pennsylvania,” said Matthew Maltese, PhD, the Director of Biomechanics Research in the Department of Anesthesiology and Critical Care Medicine at CHOP, and the executive director and principal investigator of the PPDC. “This unification of Pennsylvania’s biomedical ecosystem expands the PPDC’s network of expertise in supporting the development of much-needed devices for children.”
William Wagner, PhD, director of the McGowan Institute, added, “We are extremely excited to offer our resources and expertise as we work together with UPMC Children’s Hospital of Pittsburgh, Children’s Hospital of Philadelphia, and sciVelo to develop medical device technologies for children with particular unmet medical needs.”
For additional details please click here.
Dr. John Kellum Leads Study on Information from Urine Output in ICU Patients

McGowan Institute for Regenerative Medicine affiliated faculty member John Kellum, MD—Professor in the Departments of Critical Care Medicine, Medicine, Bioengineering, and Clinical and Translational Science at the University of Pittsburgh and the Director of the Center for Critical Care Nephrology and the Vice-Chair for Research, both appointments in the Department of Critical Care Medicine—is the lead investigator on a pilot study for the RenalSense® Clarity RMS® critical care monitoring system being conducted at the University of Pittsburgh Medical Center (UPMC).
The objective of the study is to assess the contribution of the Clarity RMS system towards improving the nursing workflow in the intensive care unit (ICU). The first part of the two-stage study launched in July 2019 was completed in 200 patients. In the second stage, data will be collected and analyzed from an additional 2000 patients.
Dr. Kellum commented, “We’ve known for years that urine output information is essential for management of critical care patients. The time has come for urine output to be monitored electronically in real-time, as is the standard practice for other vital signs in the ICU.”
Avi Kleiman, RenalSense’s Co-founder and CEO added: “We are excited to conduct this trial with Professor Kellum and the ICU team at UPMC and are hopeful that the data will strengthen the case for incorporating real-time urine output into the nursing workflow and ICU standard of care, as is done for heart rate, blood pressure and blood gases.”
RenalSense® is a privately owned medical device company dedicated to real-time renal diagnostics. The company’s first product, Clarity RMS®, provides continuous, automatic monitoring of urine flow, enabling better patient care and ICU economics. RenalSense’s next generation products will provide additional real-time parameters and expanded diagnostic capabilities, to further improve the practice of ICU and critical care management.
Changing Frequencies: Pitt Bioengineers Look Deeper into How Electrical Stimulation Activates Neurons
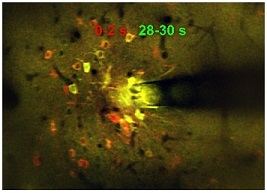
Electrical stimulation of the brain is common practice in neuroscience research and is an increasingly common and effective clinical therapy for a variety of neurological disorders. However, there is limited understanding of why this treatment works at the neural level. A paper published by McGowan Institute for Regenerative Medicine affiliated faculty member Takashi Kozai, PhD, assistant professor of bioengineering at the University of Pittsburgh Swanson School of Engineering, addresses gaps in knowledge over the activation and inactivation of neural elements that affect the desired responses to neuromodulation.
The article, “Calcium activation of cortical neurons by continuous electrical stimulation: Frequency dependence, temporal fidelity, and activation density,” was published in Neuroscience Research. Co-investigator is Kip Ludwig, PhD, associate professor of biomedical engineering at the University of Wisconsin-Madison.
For this study, Dr. Kozai’s group – the BIONIC Lab – used in vivo two-photon microscopy to capture neuronal calcium activity in the somatosensory cortex during 30 seconds of continuous electrical stimulation at varying frequencies. They imaged the population of neurons surrounding the implanted electrode and discovered that frequency played a role in neural activation – a finding that conflicted with earlier studies.
“Electrical stimulation has a large number of parameters that can be used to activate neurons, such as amplitude, pulsewidth, waveform shapes, and frequency,” explained Dr. Kozai. “This makes it difficult to compare studies because different stimulation parameters are used in other studies. Based on the parameters that were previously employed, it was thought that activation occurs in a sphere centered around the electrode where neurons near the electrode would activate more than neurons far from the electrode.
“Recent research, however, shows that stimulation mostly activates distant neurons whose axons are very close to the electrode by transmitting action potentials backward to the neuron cell body,” he continued. “We demonstrate that both of these things can be true depending on stimulation frequency and duration.”
According to Dr. Kozai, the fact that researchers can use varying stimulation parameters to activate different neurons in the same location has huge implications in basic science research. The findings will allow them to activate different neural circuits with the same implant to elicit different behaviors. Beyond its research applications, Dr. Kozai believes that this knowledge may also help in clinical settings.
“Empirical evidence in the field suggests that frequency plays a role in deep brain stimulation, but the why and how have puzzled scientists since the beginning,” said Dr. Kozai. “This research is a first glimpse into understanding the mechanisms underlying the role of frequency in clinical therapies. In the long-term, this research could also give insight on how to activate distinct glial and vascular populations, which could have a prolonged impact on behavior, attention, and tissue regeneration.”
Dr. Kozai believes that more research needs to be done to understand neuronal activation properties and hopes that this work will lead to new tools in neuroscience and improved neuromodulation therapy by explaining why electrical stimulation produces its effective responses.
Illustration: Microstimulation (130 Hz, 30 s) in a mouse cortex produced a spatiotemporal response, activating a sparse population of neurons only at the onset of stimulation (red), and a dense population of neurons throughout the stimulation duration (yellow). Photo credit: NJ Michelson & TDY Kozai/BionicLab.
Will a Patent Become a Product?

Christopher Bettinger, PhD, associate professor of materials science and biomedical engineering at Carnegie Mellon University (CMU) and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, spends much of his time thinking about how to improve the ways we take medicine. Dr. Bettinger recently spoke with Benjamin Mikek of the Pittsburgh Post-Gazette for its occasional series, “Patented in Pittsburgh,” about his work on an oral, electrical method of drug delivery and the associated patent process. Excerpts from that article follow.
Dr. Bettinger has been working for several years at CMU on ways to improve the materials and systems that help deliver medicine to the human body.
For decades, large capsules have been a popular method for delivering helpful chemicals to the stomach. But they quite literally fall apart when a medicine needs to be delivered to the lower part of the digestive tract.
The acidic stomach destroys nearly everything that enters, while the intestines are usually not caustic enough to dissolve the capsules’ heavy chemical armor needed to survive the stomach. Even when a pill can deliver something to the small intestine, it’s hard to predict exactly when and where it will finally dissolve.
To create an effective — and more predictable -— solution for medicines’ hazardous trips, Dr. Bettinger turned to electronics.
“The vision here is a class of medical devices that is electrified,” he said.
As part of that plan, Dr. Bettinger filed for a patent on an “ingestible, electrical device for oral delivery of a substance” in October 2013. A patent was finally issued February of this year.
Though the patent lists Dr. Bettinger as the inventor, it primarily belongs to his employer, CMU. Like most research-driven universities, the Oakland institution claims the intellectual property rights arising from the work that it supports.
University ownership of patents might not sound like the best deal for researchers, but many faculty members would prefer to focus on research, rather than production.
“I tend to not be interested in the commercialization of these things myself,” Dr. Bettinger admitted. “Patenting … is part of the game that we faculty play now.”
At CMU, researchers who discover something that may qualify for a possible patent disclose their invention to the university, which then hires outside attorneys to handle most of the application process.
“Our conversation, internally, is to see whether something should be patented or not, then we work with lawyers,” said Dr. Bettinger.
Before the lawyers get involved, though, invention takes a lot of work. Dr. Bettinger estimated that he spent about two years working in the lab to perfect the device — and that’s before any commercialization or safety testing.
Use of the technology in patients is still far in the future but work by other scientists indicates that the microbiome — basically the colonies of bacteria living in people’s digestive systems — could play a role in everything from diabetes to the effectiveness of cancer treatment.
Millions of dollars are devoted to treating those diseases, meaning the patent could eventually pay dividends for its owner.
Those dividends might help offset what Dr. Bettinger estimated to be a $300,000 investment in research.
The long path to market
Dr. Bettinger’s invention is far from the commercialization stage.
He said that matters of toxicity, regulatory approval by the U.S. Food and Drug Administration, the cost of production and the decision about what types of medicine to deliver would all need to be sorted out by a potential licensee.
His project was directly sponsored by the university, so the school owns the patent. He’s not dismayed.
“I think it’s just the way it is,” he said. “It’s a very reasonable arrangement.”
First Patient Dosed with VY-AADC Gene Therapy in Parkinson’s Phase 2 Trial

Voyager Therapeutics, Inc., a clinical-stage gene therapy company focused on developing life-changing treatments for severe neurological diseases, announced dosing of the first patient in RESTORE-1, a Phase 2, randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of VY-AADC for the treatment of Parkinson’s disease in patients with motor fluctuations that are refractory to medical management.
“Patients with Parkinson’s disease need new therapeutic options, especially as the disease progresses and there is less AADC enzyme in parts of the brain where it is needed to convert levodopa to dopamine,” said McGowan Institute for Regenerative Medicine affiliated faculty member R. Mark Richardson, MD, PhD, Associate Professor, Director of Epilepsy and Movement Disorders Surgery at the University of Pittsburgh Medical Center and principal investigator in the RESTORE-1 trial. “Voyager’s VY-AADC is an experimental gene therapy that is designed to deliver the AADC gene into brain cells where the enzyme can be produced to increase dopamine production. We are excited to contribute to this Phase 2, placebo-controlled trial of VY-AADC in patients with Parkinson’s disease.”
The Phase 2 RESTORE-1 trial will enroll patients who have been diagnosed with Parkinson’s disease for at least four years, are not responding adequately to oral medications, and have at least three hours of OFF time during the day as measured by a validated self-reported patient diary. Patients who meet the eligibility criteria will be randomized (1:1) to one-time administration of VY-AADC (for a total dose of up to 2.5×1012 vector genomes) or placebo surgery.
The primary endpoint of RESTORE-1 is ON time without troublesome dyskinesia, or good ON time, as measured by a self-reported patient diary at 12 months. Secondary endpoints include diary OFF time, other motor function and quality of life measures from the United Parkinson’s Disease Rating Scales (UPDRS-II-III scores), the Parkinson’s Disease Questionnaire (PDQ-39), and patient’s global function as measured by the proportion of participants with improvement on the Clinical Global Impression (CGI) score. The trial will also measure non-motor symptoms from the Non-Motor Symptom Scale (NMSS), as well as safety.
Parkinson’s disease is a chronic, progressive and debilitating neurodegenerative disease that affects approximately 1,000,000 people in the U.S.1 and seven to 10 million people worldwide2. While the underlying cause of Parkinson’s disease in most patients is unknown, the motor symptoms of the disease arise from a loss of neurons in the midbrain that produce the neurotransmitter dopamine. Declining levels of dopamine and the enzyme needed to convert levodopa to dopamine in this region of the brain, the putamen, leads to the motor symptoms associated with Parkinson’s disease including tremors, slow movement or loss of movement, rigidity, and postural instability. Additional motor symptoms during the advanced stages of the disease include falling and difficulty with speech and swallowing, with patients often requiring the daily assistance of a caregiver.
1 Willis et al, Neuroepidemiology. 2010;34:143–151.
Dance Party Raises Awareness of Needed Global Vision Medical/Surgical Care

McGowan Institute for Regenerative Medicine affiliated faculty member Jenny Yu, MD, Vice Chair of Clinical Operations for the Department of Ophthalmology, UPMC Eye Center, a member on the Orbital, Oculoplastic, and Aesthetic Surgery Service, and an Assistant Professor of Ophthalmology and Otolaryngology, University of Pittsburgh, is a co-founder of Project Theia along with Katie Duncan, MD, MDEyecare LLC. Founded in 2017, Project Theia is a non-profit organization named after the Greek goddess of sight and heavenly light and is focused on
- Providing free medical and surgical care to the under-served global community within the scope of oculoplastic, reconstructive, orbital and facial surgery.
- Educating surgeons in under-served global communities extending best practices for oculoplastic, reconstructive, orbital and facial surgery. The education component of Project Theia’s work serves to create a sustainable model for continued quality care in these regions.
- Connecting industry with these developing countries to provide the necessary tools, devices, and equipment for the ongoing delivery of quality care.
Both Drs. Yu and Duncan are fellowship trained oculoplastic surgeons who are experienced in trauma and reconstructive surgeries. The Project Theia team also includes other facial plastic surgeons, ophthalmic surgeons, nurses, and anesthetists. For each country of service, the members of the team will vary based on the local needs. To date, some of the countries Project Theia has travelled to include Ghana, India, and Mexico.
Recently, a 1980s’ themed dance party was held in Pittsburgh to raise awareness and funds for Project Theia. The event raised more than $30,000.
“We wanted to create something that could fill a niche. When a child, for example, is born with a drooping eyelid at birth and it is not taken care of, they can lose vision permanently because of that. We have the ability to change that outcome, and we felt an obligation to do so,” said Dr. Duncan.
Added Dr. Yu: “We’ve traveled to India, Mexico, Kenya and more. It is so gratifying to be able to help people live their best lives. It’s what keeps us motivated to continue.”
AWARDS AND RECOGNITION
UPMC Sports Medicine Building Renamed for Dr. Freddie Fu

In honor of the over more than 30 years of pioneering work, unwavering dedication, and commitment to UPMC Sports Medicine by Freddie Fu, MD, the medical building on the campus of the UPMC Rooney Sports Complex has been renamed the UPMC Freddie Fu Sports Medicine Center.
Dr. Fu founded UPMC’s sports medicine program in 1986. It was housed in a 1,500-square-foot suite in the Iroquois Building in Oakland. Under his leadership, UPMC Sports Medicine quickly grew to become one of the largest, most comprehensive clinical and research programs of its kind in the world, uniquely placing multiple specialists under one roof—including primary care sports medicine physicians, orthopaedic surgeons, physical therapists, athletic trainers, nutritionists, neuropsychologists and others—working together toward preventing and treating a full range of chronic and acute sports-related and non-sports-related injuries and conditions ranging from ankle sprains to knee ligament tears to concussions.
As the first team physician for Mount Lebanon and Central Catholic High Schools in 1984, Dr. Fu spearheaded the first high school athletic trainer program in western Pennsylvania, a program that has grown into one of the largest in the country with 44 high schools, seven with onsite team physicians. In the same year, Dr. Fu became the company physician for Pittsburgh Ballet Theatre, a relationship that still exists today.
In 2000, after outgrowing its second location at Baum Boulevard and North Craig Street, the center moved to a new 37,000-square-foot facility on Pittsburgh’s South Side in what is now the UPMC Rooney Sports Complex. Partly designed by Dr. Fu, the state-of-the-art complex houses the indoor and outdoor training facilities of the University of Pittsburgh Panthers and the Pittsburgh Steelers.
For 32 years, Dr. Fu has served as the head team physician and orthopaedic surgeon for the University of Pittsburgh athletic department. He has been the David Silver Professor and chair of the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine since 1997. He is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Dr. Fu has worked momentously over the years to continue to set the bar higher not only for UPMC, but also for the rest of the world. He established the University of Pittsburgh’s Sports Medicine Fellowship Program, which attracts physicians from around the globe to learn surgical techniques and conduct research with Dr. Fu and his colleagues in the Department of Orthopaedic Surgery.
Dr. Fu and his multi-specialty teams have built clinical excellence with evidence-based treatments, published in an endless number of scientific journals, setting a standard of care replicated by surgeons around the world. He is an author of more than 600 peer-reviewed articles and has made over 1,200 national and international presentations, co-authored 173 book chapters and edited 30 major orthopaedic textbooks.
Dr. Fu’s intense work has helped revolutionize anterior cruciate ligament (ACL) reconstruction and he is extoled specifically for his relentless scientific research and clinical expertise in treating this injury, common in athletes of all ages and skill levels. His reputation has made the UPMC Sports Medicine program the destination of hope for Olympic, collegiate and professional athletes when their careers are in jeopardy and a marquis program for nonathletic patients who need treatment and rehabilitation to safely return to their life’s activities.
In July 2016, Dr. Fu was inducted into the American Orthopaedic Society for Sports Medicine (AOSSM) Hall of Fame, selection into which is one of the Society’s highest honors with only a select few inductees named each year. In recognition, the council of the City of Pittsburgh dedicated September 13, 2016, to be Dr. Freddie Fu Day.
Because of Dr. Fu’s medical achievements and their impact locally and internationally, along with his many contributions to enrich the Pittsburgh community, Pittsburgh Magazine named him one of the 100 most influential Pittsburghers of the 20th century in 1999. He is consistently listed in the magazine’s annual “Best Doctors” issue and has earned a place among Pittsburgh’s most beloved residents.
Congratulations Dr. Fu!
Illustration: Dr. Fu poses near new building signage. UPMC.
Pittsburgh Business Times Selects Jelena Janjic, PhD, for 2018 Innovator Award

Duquesne University Associate Pharmacy Professor Jelena Janjic, PhD, received a 2018 Innovator Award from the Pittsburgh Business Times for her research in pain nanomedicine. Dr. Janjic is an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
The founder and co-director of Duquesne’s Chronic Pain Research Consortium, Dr. Janjic with her team of students and collaborators from Duquesne, the University of Pittsburgh, the University of Texas in Dallas, Wake Forest University and the U.S. Air Force created the first inflammatory pain nanomedicine that could significantly reduce the need for opioids in treating pain. Dr. Janjic is an Oak Ridge Institute for Science and Education faculty fellow and principal scientist with the 59th Medical Wing of the Air Force.
Dr. Janjic and her team found that nanomedicines, which carry miniscule amounts of drugs, reduced pain after a single injection for more than a week in rats and other animals. The nanomedicines carry 2,000 times less medicine than a typical dose, which could reduce the need for opioids in treating various types of pain, including after-injury, surgery or even cancer.
The Pittsburgh Business Times Innovator Awards recognize individuals who have made extraordinary advances in their respective fields by challenging conventional thinking and developing products and new approaches that can change the world.
Dr. Janjic and other Pittsburgh innovators were honored at an awards ceremony on December 5.
Congratulations, Dr. Janjic!
Boninger Lab Student Receives Clinical and Translational Science Postdoctoral Fellowship

Stephanie Rigot, DPT, a physical therapy and bioengineering graduate student at the University of Pittsburgh, received a Postdoctoral Clinical and Translational Science Fellowship. This competitive award provides a stipend and partial tuition support for up to two years of multidisciplinary clinical and translational research. Dr. Rigot works in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD, Professor and UPMC Endowed Vice Chair for Research in the Department of Physical Medicine & Rehabilitation.
Dr. Rigot is a member of the inaugural cohort of the Doctor of Physical Therapy/PhD in Bioengineering (DPT-PhD) dual-degree program, a unique offering that integrates clinical and research experiences in the School of Health and Rehabilitation Sciences and the Swanson School of Engineering.
“This program combines the outstanding evidence-based physical therapy education and innovative bioengineering research training that already exists at the university and builds upon synergies between faculty members of the nationally-ranked Departments of Bioengineering and Physical Therapy,” said Patrick Sparto, associate professor of physical therapy and co-director of the DPT-PhD program.
In the Boninger Lab, Dr. Rigot aims to create a new clinical prediction rule for a patient’s ambulatory ability after spinal cord injury using lower limb movement measured by activity monitors. Current prediction rules, she explained, are not sufficient to predict ambulation for individuals with moderate impairments and do not provide the full picture for a patient’s mobility potential. These shortcomings can lead to improper use of therapy time and limit an individual’s functional independence.
“These tools fail to provide insight into the quality of gait or whether it is likely to be a functional mode of mobility,” Dr. Rigot said. “By using machine learning techniques, we aim to combine clinical measures, psychosocial and environmental factors, and lower limb movements to develop prediction models that provide improved insight into the long-term mobility prognosis of individuals with acute spinal cord injuries. We hope that the models can be used to optimize patient care during rehabilitation,” she explained.
Dr. Rigot graduated with a Doctor in Physical Therapy degree in April 2018 and is now focusing on the bioengineering aspects of the program to complete her PhD.
Dr. Boninger, who chairs her doctoral committee, said, “Stephanie and her project embody what the DPT-PhD program hoped to achieve. She is completing highly technical research that is clinically relevant and immediately translatable into practice. I am not surprised she was selected for this highly competitive grant – she is an outstanding student working in an important area.”
Illustration: University of Pittsburgh Department of Physical Medicine & Rehabilitation.
McGowan Staff Receive Service Awards

Dr. William Wagner, Director of Institute presented length of service awards to the following employees: (L to R) Laura Miller. PhD (5 years), Julia Bennett (5 years), Teri Dulak (10 years), and Dr. Wagner. Not pictured: Rebecca Bauroth (10 years), Patrick Cantini (10 years), Michele Krugh (5 years), and Lori Walton (10 years).
Important Upcoming Dates
McGowan Institute Annual Retreat: March 11-12, 2019
Registration is now open!
Click Here for more information
McGowan Institute Open-to-the-Public Forum: March 12, 2019
“The Hope vs. Hype of Regenerative Medicine”
Click Here to register
ASAIO 65th Annual Conference
Abstract Submission Deadline is February 7th, 2019
Click Here to submit
ASAIO Student Design Competition
Deadline for Preliminary Proposals is February 11, 2019
Click Here for competition guidelines
Regenerative Medicine Summer School
Registration is now open!
Click Here for more information
Pittsburgh Life Sciences Week: May 13-17, 2019
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#192 –– Dr. Angela Panoskaltsis-Mortari discusses her research in bioengineering autologous tissues such as trachea and esophagus using 3D bioprinting and customized hydrogels. She also discusses her connection with the Journal of Immunology and Regenerative Medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Michelson NJ, Eles JR, Vazquez AL, Ludwig KA8, Kozai TDY
Title: Calcium activation of cortical neurons by continuous electrical stimulation: Frequency dependence, temporal fidelity, and activation density
Summary: Electrical stimulation of the brain has become a mainstay of fundamental neuroscience research and an increasingly prevalent clinical therapy. Despite decades of use in basic neuroscience research and the growing prevalence of neuromodulation therapies, gaps in knowledge regarding activation or inactivation of neural elements over time have limited its ability to adequately interpret evoked downstream responses or fine-tune stimulation parameters to focus on desired responses. In this work, in vivo two-photon microscopy was used to image neuronal calcium activity in layer 2/3 neurons of somatosensory cortex (S1) in male C57BL/6J-Tg(Thy1-GCaMP6s)GP4.3Dkim/J mice during 30 s of continuous electrical stimulation at varying frequencies. We show frequency-dependent differences in spatial and temporal somatic responses during continuous stimulation. Our results elucidate conflicting results from prior studies reporting either dense spherical activation of somas biased toward those near the electrode, or sparse activation of somas at a distance via axons near the electrode. These findings indicate that the neural element specific temporal response local to the stimulating electrode changes as a function of applied charge density and frequency. These temporal responses need to be considered to properly interpret downstream circuit responses or determining mechanisms of action in basic science experiments or clinical therapeutic applications.
Source: J Neurosci Res. 2018 Dec 26. [Epub ahead of print]
GRANT OF THE MONTH
PI: Michael Boninger
Title: A Biomimetic Approach Towards a Dexterous Neuroprosthesis
Description: Cervical spinal cord injury results in the loss of arm and hand function, which significantly limits independence and results in costs over the person’s lifespan. A brain-computer interface (BCI) can be used to bypass the injured tissue to enable control of a robotic arm and to provide somatosensory feedback. Two primary limitations of current state-of-the-art BCIs for arm and hand control are: (1) the inability to control the forces exerted by the prosthetic hand and (2) the lack of somatosensory feedback from the hand. In the proposed study, we seek to considerably improve dexterous control of prosthetic limbs by implementing decoding strategies that enable the user to not only control the movements of the arm and hand, but also the forces transmitted through the hand. We anticipate that our biomimetic approach to decoding will yield intuitive, dexterous control of the prosthetic hand. Tactile sensations will be conveyed to the user through intracortical microstimulation (ICMS) of somatosensory cortex. The spatiotemporal patterns of stimulation will be based on our basic scientific understanding of how tactile information is encoded in somatosensory cortex, which we expect will result in more natural and intuitive sensations. In order to achieve our goal of developing a dexterous neuroprosthesis, we have brought together a team with human BCI experience from the University of Pittsburgh along with the basic science expertise at both Pitt and the University of Chicago. We will collaborate with experts in implantable neurotechnology (Blackrock Microsystems) and robotics (The Biorobotics Institute) to ensure that the device hardware allows us to take a biomimetic approach for control and feedback with an eye toward clinical translation. A total of 4 participants will be tested in a multisite study to accomplish the following three specific aims. Aim 1: Evoke natural and intuitive tactile sensations through ICMS of somatosensory cortex. We expect that biomimetic ICMS will evoke sensations that more closely resemble everyday tactile sensations and intuitively convey information about contacted objects than does standard fixed-frequency ICMS. Aim 2: Derive kinematic and kinetic signals from motor cortex for hand control. We will assess the degree to which motor cortical neurons encode forces exerted on objects. Based on these observations, we will develop hybrid decoders that enable controlling both the movement and force using a synergy-based approach. Aim 3: Demonstrate improved arm and hand function with a biomimetic sensorimotor BCI that combines the sensory feedback developed in Aim 1 with the hybrid decoding developed in Aim 2. A battery of functional assessments will be used including novel metrics designed specifically for sensorimotor prosthetics along with well-established tests identified in the NIH Common Data Elements. We anticipate that subjects will substantially improve their dexterity using a biomimetic BCI as compared to non-biomimetic BCIs or BCIs without somatosensory feedback.
Source: National Institute of Neurological Disorders and Stroke
Term: September 30, 2018 – August 31, 2019
Amount: $1,330,521
