December 2016 | VOL. 15, NO. 12| www.McGowan.pitt.edu
Dr. William R. Wagner Named Fellow of the National Academy of Inventors

William R. Wagner, PhD, Director of the McGowan Institute for Regenerative Medicine and Professor of Surgery, Bioengineering & Chemical Engineering at the University of Pittsburgh, has been named a Fellow of the National Academy of Inventors (NAI). Election to NAI Fellow status is the highest professional distinction accorded solely to academic inventors who have demonstrated a prolific spirit of innovation in creating or facilitating outstanding inventions that have made a tangible impact on quality of life, economic development, and the welfare of society. Academic inventors and innovators elected to the rank of NAI Fellow status have been nominated by their peers for outstanding contributions to innovation in areas such as patents and licensing, innovative discovery and technology, significant impact on society, and support and enhancement of innovation. The 2016 Fellows Gala and Induction Ceremony will take place as part of the NAI 6th Annual Conference on April 6, 2017, in Boston.
Dr. Wagner is chairman for the Tissue Engineering and Regenerative Medicine International Society (TERMIS) Americas and past-president of the American Society for Artificial Internal Organs (ASAIO). He is also the Founding Editor and Editor-in-Chief of one of the leading biomaterials journals, Acta Biomaterialia, and serves on the editorial boards of several journals in the biomedical engineering and regenerative medicine fields. Dr. Wagner’s innovations in the fields of cardiovascular devices and tissue engineering have been recognized by awards such as the Society for Biomaterials Clemson Award for Applied Research, the TERMIS Senior Scientist Award, selection to the “Scientific American 50,” the Chancellor’s Distinguished Research Award, and University of Pittsburgh Innovator Awards in 2007, 2008, 2009, 2010, and 2014 for licensing activity associated with his patents and patent filings.
Dr. Wagner holds 17 issued patents and an additional 25 patent filings in the field of cardiovascular biomaterials, medical devices, imaging, and tissue engineering. Five of his patents have been licensed by the University Office of Technology Management. Together with collaborator Dr. David Vorp and two former students, Dr. Wagner co-founded a company, Neograft Technologies, that has raised over $23M in funding to date. The company recently initiated clinical trials in Europe around an invention that creates a temporary elastic support system around veins prior to their use as arterial grafts in coronary artery bypass surgery. With the research group that he leads, Dr. Wagner has invented a series of new biomedical materials focused on biodegradable, elastic polymers that can be used to slow the dilatation of the heart following heart attack as well as in other applications, such as creating cardiac valves.
Under Dr. Wagner’s direction, the McGowan Institute is a leader in the commercialization of medical device and regenerative medicine technologies and has made an international impact on healthcare with its development of circulatory assist devices (including the current most commonly implanted ventricular assist device), pulmonary assist devices, and extracellular matrix based materials for regenerative repair and healing. He has played an important role in the facilitation and nurturing of academic innovation and the transfer of this technology to positively impact society. The McGowan Institute has created 26 start-up companies that have attracted over $500M in external investment. The McGowan Institute is also noteworthy for its numerous programs to aid its faculty in rapidly refining their innovation efforts by providing ready access to an executive-in-residence program for competitive landscape evaluation and business model development. This program, which has been supported by philanthropy and the University of Pittsburgh Innovation Institute has refined numerous nascent technologies and helped to refine business plans and technical approaches. The Institute has also been well ahead of its peers by its early integration of regulatory expertise into product development efforts. This acumen has aided the Institute as it has attracted major research and technology development grants from the Department of Defense in creating solutions for warfighter injuries, and in its partnering with industrial partners in the medical device and pharmaceutical space.
Congratulations, Dr. Wagner!
RESOURCES AT THE MCGOWAN INSTITUTE
Trichrome Stains
Trichrome Stains are intended for use in the study of connective tissue, muscle and collagen fibers. Trichrome stains are used primarily for distinguishing collagen from muscle tissue.
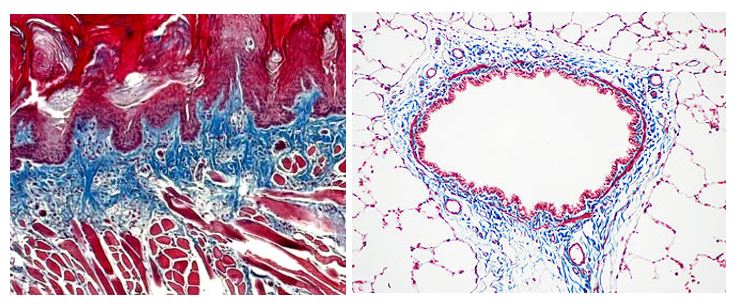
The McGowan Institute Histology Core Laboratory offers Masson Trichromes, automated staining results in one week or less, beautifully reproducible.
Through the whole month of January you will receive 25% off your trichrome stains, when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials by email or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Did you know the more samples you submit to the histology lab the less you pay per sample? Contact Lori to find out how!
SCIENTIFIC ADVANCES
DOD Grant Received to Accelerate Bone Healing
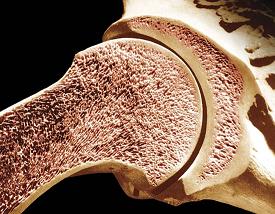
Military personnel are substantially burdened with traumatic bone injury to the extremities, but no ideal therapy is available to regenerate large bone volumes in compromised wounds. These wounds are sub-optimal for regeneration because the vascular damage and immune response provoke oxygen deficiency and inflammation which impair bone growth and drive formation of fibrous tissue.
A Department of Defense (DOD) grant for $2.1M (1.7M direct) over 3 years to accelerate bone healing has been awarded to University of Pittsburgh’s Center for Craniofacial Regeneration (CCR) / McGowan Institute for Regenerative Medicine and the U.S. Army Institute of Surgical Research (ISR). The project’s team of McGowan Institute affiliated faculty members includes:
- Juan Taboas, PhD, Assistant Professor with the Department of Oral Biology in the School of Dental Medicine and the Department of Biomedical Engineering in the Swanson School of Engineering, PI
- Alejandro Almarza, PhD, Associate Professor in Oral Biology in the School of Dental Medicine at the University of Pittsburgh with a secondary appointment in the Department of Bioengineering, CoI
- Yadong Wang, PhD, William Kepler Whiteford Professor in Bioengineering with adjunct positions in Chemical Engineering, Mechanical Engineering and Materials Science, and Surgery at the University of Pittsburgh, CoI
The team’s technology to accelerate bone healing is an off-the-shelf biologic device that can be loaded with minimally manipulated autologous mesenchymal stem cells (MSCs) at the point-of-care. It consists of the following components:
- drugs to control the immune response and promote healing
- hydrogel carrier to deliver the drugs and to control tissue formation by MSCs
- nanoparticles to deliver the drugs over prolonged periods
- porous scaffold to provide mechanical support in large bone defects
These components are assembled in two forms depending on the nature of the bone injury: an injectable hydrogel and an implantable hydrogel infused scaffold. The team expects that an injectable device (components 1-3) will be used by first responders to stabilize bone fragments in comminuted fractures, prevent fibrous tissue ingrowth, and rapidly initiate regeneration. They expect that an implantable device (all components) will be used in the operating theater to promote bone formation and minimize non-union when conventional grafts and bone fillers are contraindicated. They will evaluate bone regeneration over time in two bone injury models in swine, and will test the injectable device in a simulated comminuted fractures of the fibula while the implantable device in large fibular bone defects. Also, they will evaluate bone formation and strength, revascularization and reinnervation, and the local and host immune responses.
Approximately 20% of injured combat personnel suffer extremity bone fracture and loss, of which 80% are open compromised wounds with significant tissue loss post-debridement. The team’s device will minimize the severe morbidity and the treatment cost for wounded military personnel, and will improve their quality of life and return to service. Ultimately, this work will translate to several clinical therapies in the form of pharmacological interventions and therapeutic devices to promote skeletal healing. These will decrease clinical involvedness and impact individual’s lives by speeding skeletal healing, diminishing non-union and tissue fibrosis incidence, and reducing multi-surgery procedures. This work will have a similar strong impact for non-military applications as well.
Illustration: Longitudinal section of the humerus (upper arm bone). Dr. Don Fawcett—Visuals Unlimited/Getty Images.
Looking for Ways to Mend a Broken Heart

Many lower forms of life on earth exhibit an extraordinary ability to regenerate tissue, limbs, and even organs—a skill that is lost among humans and other mammals. Now, a University of Pittsburgh researcher has used the components of the cellular “scaffolding” of a zebrafish to regenerate heart tissues in mammals, specifically mice, as well as exhibiting promising results in human heart cells in vitro.
The findings, published in Science Advances, offer promise to overcoming heart disease, the leading cause of death for men and women.
The study, led by McGowan Institute for Regenerative Medicine faculty member Yadong Wang, PhD, the William Kepler Whiteford Professor in Bioengineering in the Swanson School of Engineering and the principal investigator of the Biomaterials Foundry at Pitt, found that a single administration of extracellular matrices (ECM) from zebrafish hearts restored the function of the heart and regenerated adult mouse heart tissues after acute myocardial infarction.
“The heart beats as if nothing has happened to it,” said Dr. Wang. “And our approach is really simple.”
The study also found that the zebrafish ECM protected human cardiac myocytes—specialized cells that form heart muscle—from stresses.
ECM are the architectural foundations of tissues and organs; not only do they provide a “scaffolding” on which cells can grow and migrate, they assist in the signaling necessary for the organ to develop, grow, or regenerate.
In mammals, the heart quickly loses the ability to regenerate after the organism is born, except for a brief period after birth. In lower animals, such as zebrafish, the heart retains that ability throughout their lives: up to 20 percent of a zebrafish’s heart can be damaged or removed, and within days the heart’s capacity has been fully restored.
Dr. Wang and his team—which included McGowan Institute for Regenerative Medicine affiliated faculty member Kang Kim, PhD, Associate Professor of Medicine and of Bioengineering at the University of Pittsburgh and the Heart and Vascular Institute, UPMC—first separated the ECM from the cells so that the recipient heart would not reject the treatment. They did this by freezing the zebrafish cardiac tissue, causing the cell membranes to burst and allowing the researchers to retrieve the ECM, a process called decellularization. Dr. Wang noted that he and his colleagues are among the first to decellularize non-mammalian tissues for applications in regenerative medicine. They then injected the ECM into the hearts of mice with damaged heart muscles and watched the hearts repair themselves.
“It’s difficult to inject foreign cells into a body because the body will recognize them as foreign and reject them; that’s not the case with ECM,” said Dr. Wang. Dr. Wang explained that, because ECMs are composed of collagen, elastin, carbohydrates and signaling molecules, and have no cell surface markers, DNA or RNA from the donor, the recipient is less likely to reject the treatment.
Dr. Wang said that restored function starts almost immediately, and healing is noticeable as early as 5 days after treatment; within a week, his team could see the heart beating more strongly than the hearts of the untreated animals.
The researchers tested the effectiveness of ECM from normal zebrafish and from zebrafish with damaged hearts, in which the ECM had already begun the healing process. They found that while both types of ECM were effective in repairing damage to the mice hearts, the ECM obtained from the zebrafish hearts that were healing were even more potent in restoring heart function in the mice.
Dr. Wang is now working on a process to regenerate nerves in mammals using the same process and hopes to expand the heart treatments to larger animals in a future study.
Abstract (Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. William C. W. Chen, Zhouguang Wang, Maria Azzurra Missinato, Dae Woo Park, Daniel Ward Long, Heng-Jui Liu, Xuemei Zeng, Nathan A. Yates, Kang Kim, and Yadong Wang. Science Advances; 18 Nov 2016: Vol. 2, No. 11, e1600844.)
$2.95 Million NIH Grant to Predict At-Risk Cerebral Aneurysms Received
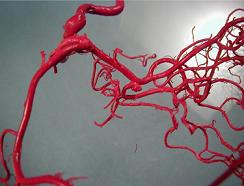
Although cerebral aneurysms affect a substantial portion of the adult population, the risk of treatment including open brain surgery often outweighs the risks associated with rupture. With increasing numbers of unruptured aneurysms detected using noninvasive imaging techniques, there is an urgent need for a reliable method to distinguish aneurysms vulnerable to impending rupture from those that are presently robust and can be safely monitored. An international research team led by the University of Pittsburgh Swanson School of Engineering recently received a grant from the National Institutes of Health (NIH) to improve risk assessment and treatment of this devastating disease.
Principal investigator of the 5-year, $2,950,622 grant, “Improving cerebral aneurysm risk assessment through understanding wall vulnerability and failure modes,” is McGowan Institute for Regenerative Medicine affiliated faculty member Anne M. Robertson, PhD, the William Kepler Whiteford Professor of Engineering at the Swanson School. McGowan Institute affiliated faculty member Simon Watkins, PhD, Distinguished Professor of Cell Biology and Director of Pitt’s Center for Biological Imaging, is a co-investigator on the study. The R01 grant is funded through the NIH National Institute of Neurological Disorders and Stroke.
“The cells in our blood vessels have a remarkable capacity for rebuilding and maintaining the collagen fibers that give the artery walls their strength. Unfortunately, this natural process can be derailed by the abnormal fluid flow in brain aneurysms, leading to vulnerable walls and rupture,” explained Dr. Robertson. “Understanding the factors that discriminate between robust aneurysm walls with well-organized collagen fibers, and fragile aneurysm walls with diverse changes to the collagen architecture, is essential for improving risk assessment and developing new treatments to prevent rupture.”
To support their work, Dr. Robertson and a multi-disciplinary team of world leaders in aneurysm research from Pitt, Allegheny General Hospital in Pittsburgh, George Mason University in Virginia, University of Illinois at Chicago, and Helsinki University Central Hospital and Kuopio University Hospital in Finland, will analyze brain tissue donated from patients with cerebral aneurysms. Using state-of-the-art facilities for biomechanical analysis and bioimaging, the investigators will specifically look at how and why some patients are naturally able to maintain a healthy aneurysm wall while the walls in other patients weaken, leaving the vulnerable to rupture. They will use computational mechanics to explore the possible multiple mechanisms by which hemodynamics alters the wall and study the mechanisms of structural failure.
“The diverse expertise in our team and our access to an unprecedented number of aneurysm tissue samples enables us to study this disease in an entirely new way,” Dr. Robertson said. “We are also able to leverage computational and experimental tools developed during our prior NIH supported program.”
Other co-investigators on the international team include:
- University of Pittsburgh: Spandan Maiti, PhD, Assistant Professor of Bioengineering
- Allegheny General Hospital: Khaled Aziz, MD, PhD, Department of Neurosurgery
- George Mason University: Juan Cebral, PhD, Professor of Bioengineering, Volgenau School of Engineering
- University of Illinois College of Medicine at Chicago: Fady Charbel, MD, Professor of Neurosurgery and Chief of Neurovascular Section; and Sepi Amin-Hanjani, MD, Professor of Neurosurgery
- Kuopio University Hospital (Finland): Juhana Frösen, MD, PhD, Department of Neurosurgery
- Helsinki University Central Hospital (Finland): Riikka Tulamo, MD, PhD, Department of Vascular Surgery
“Because of the critical importance and delicate nature of the brain, surgical treatment of cerebral aneurysms is avoided unless absolutely necessary. That’s why doctors and surgeons need a more effective way to determine whether a patient with a cerebral aneurysm is at risk for rupture,” Dr. Robertson said. “We expect that by understanding the differences in the vulnerable and robust aneurysm wall, we will be able to improve risk assessment, identify biomarkers of wall fragility, and provide essential knowledge for developing pharmacological treatments to harness and augment the natural repair process of the aneurysm wall.”
Illustration: Cast of human brain vessels showing cerebral aneurysm in center, taken by Charles Kerber, MD.
Pitt Researchers Collaborate to Develop Lifesaving “Rescue Stent”

According to a study published in the Journal of Surgical Research, more than 80 percent of people who suffer traumatic injury to a major artery or vein die from rapid blood loss. The window for saving lives of people with other potentially fatal afflictions may be hours, days, or even weeks, but the outcome of a non-compressible hemorrhage within the torso is determined in mere minutes.
The United States Department of Defense has granted $2.5 million in funds for a 4-year research collaboration between the University of Pittsburgh Swanson School of Engineering and UPMC Division of Vascular Surgery. The research team will develop a removable, collapsible, and biocompatible trauma stent to prevent internal bleeding from the aorta. The “Rescue Stent” will have both military and civilian applications and could greatly reduce fatalities caused by gunshot wounds, stabbings, and other related torso injuries.
McGowan Institute for Regenerative Medicine faculty member Bryan Tillman, MD, PhD, assistant professor of vascular surgery at Pitt’s School of Medicine, will serve as principal investigator and provide clinical insight and lead the testing. Joining Dr. Tillman on the study are three engineering professors from Pitt’s Swanson School: Youngjae Chun, PhD, Sung Kwon Cho, PhD, and William Clark, PhD. Parthasarathy Thirumala, MD, co-director of the Center of Clinical Neurophysiology at UPMC, will also assist the study as a co-investigator to ensure the Rescue Stent avoids the paralysis associated with other current approaches for hemorrhage control.
“If there is internal bleeding, applying pressure to the wound won’t stop it,” said Dr. Chun. The current treatment involves essentially placing a balloon somewhat randomly inside the patient’s artery to block blood loss. However, use of the balloon can result in organ failure and paralysis because it causes a complete stoppage of blood flow. In about 4 minutes, we can implant our stent, redirect blood flow, and stabilize a patient.”
McGowan Institute for Regenerative Medicine affiliated faculty member Dr. Chun, assistant professor in the Departments of Industrial Engineering and Bioengineering, will be responsible for designing, modeling, and fabricating the stent. He will investigate various design methods and advanced manufacturing processes to create functional rescue stents, including geometric/stress analyses, micro laser welding, thermal treatment, mechanical-chemical joining processes, and biocompatible surface treatments.
Dr. Cho, associate professor of mechanical engineering and materials science, will work on the fabrication of radio-frequency identification (RFID) and vital sign monitoring sensors. The RFID sensor—which is wireless, inexpensive, and more portable than the equipment used for internal positioning in hospitals—will allow Dr. Cho to position the device inside the body without X-rays or ultrasound imaging.
“An RFID sensor can be used to make sure we position the stent exactly at the point of trauma without restricting blood flow from the undamaged blood passageways,” said Dr. Cho. “It is the same technology used in a grocery store scanner; and an emergency room physician, general surgeon, or resident can easily track the stent to ensure it’s properly placed.”
Dr. Clark, professor of mechanical engineering and materials science, will participate in the sensor development and take the lead on their integration and data analysis, while working with Dr. Cho to make sure the sensors can be identified and interpreted once inside the body.
“In addition to accurately positioning the stent, the sensors we are developing will allow us to monitor the patient’s vitals,” said Dr. Clark. “The individual placing the stent will have a clear idea of what’s going on inside the patient and can drastically open the window of time the patient has to survive before being treated by a vascular surgeon.”
“The intersection of medicine, industrial engineering, and mechanical engineering has been very important to the development of the Rescue Stent,” added Dr. Tillman. “It requires a great deal of engineering expertise to ensure the stent is compatible with the needs of the medical community. The interdisciplinary environment at Pitt lends itself to these kinds of collaborations in a really spectacular way.”
Concussions are Treatable, More Research Needed

Concussions, often viewed by the public as dire and perplexing, can be effectively treated despite their complexity, according to experts from around the U.S. in a Statement of Agreement available online and to be published in the December issue of the journal Neurosurgery.
In October 2015, leading concussion clinicians and researchers gathered at UPMC in Pittsburgh for the “Targeted Evaluation and Active Management” (TEAM) symposium, an unprecedented meeting and white paper designed to propose and share nationally the participants’ agreement on the best practices, protocols, and active therapies for treating concussions.
The conference discussions, led by chair Micky Collins, PhD, director of the UPMC Sports Medicine Concussion Program, along with co-directors Anthony Kontos, PhD, and McGowan Institute for Regenerative Medicine affiliated faculty member David Okonkwo, MD, PhD, of UPMC and the University of Pittsburgh, resulted in the Statement of Agreement publication. The 2-day meeting was fully funded by a grant from the NFL Foundation.
“This conference was remarkable because it brought together a diverse group of leading experts in cutting-edge research and clinical treatment to approach this injury in ways that will help move concussion treatment forward,” said Dr. Kontos, research director for the UPMC Concussion Program, associate professor in the University of Pittsburgh Department of Orthopaedic Surgery.
The U.S. Center for Disease Control (CDC) estimates that as many as 4 million concussions occur each year in the U.S., and sport- and recreation-related concussions in particular have increasing incidence. Symptoms, which can be subtle and last days or weeks, include but are not limited to headache, confusion, and nausea.
“There has been only limited evidence-based guidance, particularly for primary care providers, about the active treatment of concussion,” Dr. Collins said. “This makes it difficult for clinicians to determine how best to treat patients with this injury. Many are treating patients with concussion using a uniform, rest-based approach today much the same way they did a decade ago.”
Doctors typically advise patients to rest—both the brain and body—until symptoms abate, which might require accommodations at school or work. If the injury was sustained during sports, the patient is instructed not to return to play on the same day and to gradually increase aerobic, exertion-based activity while symptoms are carefully monitored.
But, as described at the symposium and in the published Statement of Agreement, research is beginning to show active rehabilitation can help people recover more quickly and safely than simply resting.
“More research in large, multicenter trials is needed to figure out what kinds of treatments are most effective for a set of symptoms and for individual patients,” Dr. Collins said. Most importantly, we believe concussions are treatable and patients can and do get better.”
A 2015 Harris Poll of more than 2,000 U.S. adults found that 71 percent did not recognize that concussions are treatable. In the same report, 1 in 3 patients who had been diagnosed with a concussion reported receiving no prescribed treatment.
“The purpose of the UPMC symposium was to engage leading clinicians and scientists in a discussion of what we know about concussion and its treatment,” Dr. Okonkwo, Professor and Executive Vice Chair of Neurological Surgery, University of Pittsburgh, Director of Neurotrauma and of the Scoliosis and Spinal Deformity Program at UPMC, Clinical Director of the Brain Trauma Research Center, and a member of the Medical Staff for the Pittsburgh Steelers, said. “We hope to build on this effort to share the best available information to improve public understanding and guide future research.”
The authors feel the Statement of Agreement is a step forward in the field and will lead to a collaborative era.
“Over the past decade, many of us individually have accumulated quite a bit of experience about which treatments work for specific symptoms and deficits caused by concussion. We are looking forward to working together to rigorously test these treatments,” said David Brody, MD, PhD, co-author and professor of neurology, Washington University School of Medicine in St Louis.
Abstract (Statements of agreement from the targeted evaluation and active management (TEAM) approaches to treating concussion meeting held in Pittsburgh, October 15-16, 2015. Collins, Michael W. PhD; Kontos, Anthony P. PhD; Okonkwo, David O. MD, PhD; et al. Neurosurgery; Post Copyedit: October 12, 2016.)
Pitt Researchers Awarded $1.7 Million NIH Grant to “Sniff Out” Better Treatment for Lung Disease
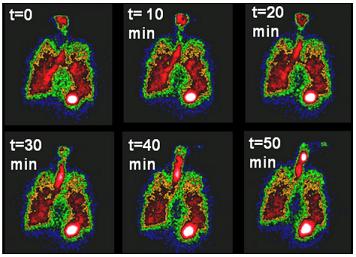
Cystic Fibrosis (CF) causes the accumulation of dehydrated mucus in the lungs which can lead to chronic infection, inflammation, and respiratory failure and drastically affects the lives of CF patients. These ever-changing complexities often make it difficult for doctors to decide which therapies will be most effective in treating the disease.
To develop better evaluation methods, the National Institutes of Health (NIH) awarded a research team at the University of Pittsburgh’s schools of engineering and medicine a highly competitive $1.7 million U01 grant to develop new mathematical models of liquid and ion transport in the human lung. These models could allow doctors to rapidly personalize interventions for patients suffering from CF and other lung diseases and administer the most effective treatment by simply studying a cell culture from the patient’s nose.
McGowan Institute for Regenerative Medicine affiliated faculty member Robert Parker, PhD, professor of chemical and petroleum engineering at the Swanson School of Engineering, and Timothy Corcoran, MD, associate professor of medicine, bioengineering and chemical engineering at the School of Medicine, in the Division of Pulmonary, Allergy and Critical Care, will lead the study as co-principal investigators. Three co-investigators will join the study: Carol Bertrand, PhD, from Pediatrics, and Joseph Pilewski, MD, and Michael Myerburg, MD, both from the Division of Pulmonary, Allergy and Critical Care Medicine.
“We know that mucus hydration and clearance are important factors in CF lung disease,” said Dr. Corcoran. “We’ve developed nuclear imaging techniques to measure how mucus and water move in the lungs. This lets us understand the individual lung pathologies of our patients and may allow us to predict what therapies will help them. The techniques we are using were actually developed here, and we’re pretty much the only ones using them.”
The researchers will begin by collecting data from patients with CF, biological parents of patients with CF who carry the CF mutation, and healthy controls. After sampling and culturing of human nasal epithelial (HNE) cells—under the direction of Dr. Myerburg—Dr. Corcoran will use aerosol-based nuclear imaging to measure mucus clearance and airway surface liquid dehydration in the lungs.
Once the researchers have collected data from the patients’ HNE cell cultures and lung imagining, they will use advanced computational techniques to find the correlation between the nasal cell physiology and lung physiology. Dr. Parker will lead the group’s effort to translate the data collected from the test subjects into multi-scale mathematical models that provide cell- and organ-level visualizations of the patients’ physiology.
“The mathematical models—through a framework of differential equations—describe how basic physiological processes contribute to experimental outcomes,” said Dr. Parker. “We can link all of the information we’ve gathered from lab experiments, physiology studies, and clinical studies to better predict how a patient will respond to different therapies. By creating millions of simulations over a broad spectrum of patients, we can identify the underlying biological mechanism and understand how the patients will respond to treatment through the painless, non-invasive sampling of the HNE cells.”
Ultimately, the researchers hope to show that nasal cell sampling and interpretation of the data by the computer models can lead to a highly personalized approach to treating a patient with CF that could begin as early as birth. This would greatly enhance a patient’s quality of life, increase life expectancy, and limit progress of the disease.
“We are always going to be limited by the number of patients we can test,” added Dr. Parker. “However, we can bridge the gap between the full set of all CF patients and a smaller set of CF patients with similar symptoms who are likely to respond to treatment in a similar way. The mathematical models will help us create those sets and let us predict outcomes and design treatments for individual patients.”
Illustration: Nuclear imaging shows mucus clearance from the lungs. These imaging techniques can be used along with systems models to help develop treatments for Cystic Fibrosis.
AWARDS AND RECOGNITION
Dr. Krzysztof Matyjaszewski Is Co-Winner of Benjamin Franklin Medal in Chemistry

The Franklin Institute in Philadelphia announced that Krzysztof Matyjaszewski, PhD, the J.C. Warner Professor of Natural Sciences at Carnegie Mellon University (CMU) and a McGowan Institute for Regenerative Medicine affiliated faculty member, and Mitsuo Sawamoto, PhD, professor of polymer chemistry at the University of Kyoto, have won its 2017 Benjamin Franklin Medal in Chemistry. They will receive the award on May 4, 2017, during a ceremony at the Franklin Institute in Philadelphia.
Dr. Matyjaszewski, a member of the chemistry faculty in CMU’s Mellon College of Science, and Dr. Sawamoto were cited “for their seminal contributions to the development of a new polymerization process involving metal catalysts. This powerful process affords unprecedented control of polymer composition and architecture, making possible new materials including improved composites, coatings, dispersants, and biomedical polymers.”
Dr. Matyjaszewski is best known for developing copper-mediated atom transfer radical polymerization (ATRP), a precise method for making macromolecules that has revolutionized the field of polymer synthesis. ATRP uses a specialized catalyst to start and stop the polymerization reaction, allowing researchers to construct polymers in a piece-by-piece fashion and precisely control their size, architecture, and function.
Since first publishing his finding on ATRP in 1995, Dr. Matyjaszewski has worked to refine the process making it more efficient and environmentally friendly. His work has been cited more than 80,000 times, making him one of the most cited chemists in the world.
Independently, Dr. Sawamoto developed a similar controlled radical polymerization process that used a different type of catalyst and activator.
Dr. Matyjaszewski has won numerous other awards, including the 2015 Dreyfus Prize in the Chemical Sciences, the 2013 AkzoNobel North America Science Award, the 2011 Wolf Prize in Chemistry, and the 2009 Presidential Green Chemistry Challenge Award. He is a fellow of the National Academy of Inventors and the American Chemical Society and a member of the National Academy of Engineering.
The Franklin Institute Awards have recognized and encouraged pre-eminent accomplishments in science and technology on an international level since the institute was founded in 1824. Past laureates include Thomas Edison, Marie Curie, Stephen Hawking, and Bill Gates.
CATER Trainee Emily Bayer – Tissue Engineering Part A Features Research Image As Cover Art

A research image by Emily Bayer – a trainee in the McGowan Institute for Regenerative Medicine Cellular Approaches to Tissue Engineering and Regeneration (CATER) Training Program – is the cover art of the recent Tissue Engineering Part A journal.
Her paper entitled, “The Influence of Platelet-Derived Growth Factor and Bone Morphogenetic Protein Presentation on Tubule Organization by Human Umbilical Vascular Endothelial Cells and Human Mesenchymal Stem Cells in Coculture,” is co-authored with Morgan Fedorchak, PhD, and McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, Chairman of the Department of Chemical and Petroleum Engineering and William Kepler Whiteford Endowed Professor in the Departments of Chemical and Petroleum Engineering, Bioengineering, Immunology, and Ophthalmology.
The cover art image is captioned: Immunofluorescent staining CD31 (red), α-SMA (green), and nuclei (blue) of a cross-section of three-dimensionally cocultured HUVECs and hMSCs showing tubule formation in response to sequential delivery of PDGF and BMP with 6 days of overlap in growth factor delivery.
Illustration: Tissue Engineering Part A.
Abstract (The Influence of Platelet-Derived Growth Factor and Bone Morphogenetic Protein Presentation on Tubule Organization by Human Umbilical Vascular Endothelial Cells and Human Mesenchymal Stem Cells in Coculture. Bayer Emily A., Fedorchak Morgan V., and Little Steven R. Tissue Engineering Part A; November 2016, 22(21-22): 1296-1304.)
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#166 –– Dr. Rocky Tuan is the Director of the Center for Cellular and Molecular Engineering, the Director of the Center for Military Medicine Research, and the Associate Director of the McGowan Institute for Regenerative Medicine. He is also a Professor in the Departments of Bioengineering and Mechanical Engineering & Materials Science at the University of Pittsburgh. Dr. Tuan discusses his research in orthopaedic research.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
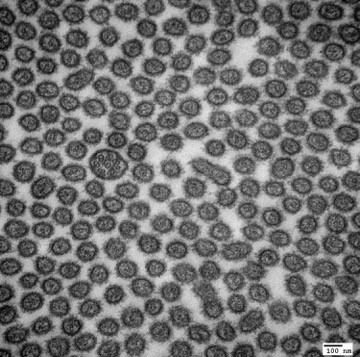
Transmission Electron Micrograph of a primary cilium within a sea of microvilli. Mouse Kidney Proximal Tubule.
Donna Stolz, PhD
Center for Biologic Imaging
PUBLICATION OF THE MONTH
Author: William C. W. Chen, Zhouguang Wang, Maria Azzurra Missinato, Dae Woo Park, Daniel Ward Long, Heng-Jui Liu, Xuemei Zeng, Nathan A. Yates, Kang Kim, and Yadong Wang
Title: Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration
Summary: Heart attack is a global health problem that leads to significant morbidity, mortality, and health care burden. Adult human hearts have very limited regenerative capability after injury. However, evolutionarily primitive species generally have higher regenerative capacity than mammals. The extracellular matrix (ECM) may contribute to this difference. Mammalian cardiac ECM may not be optimally inductive for cardiac regeneration because of the fibrotic, instead of regenerative, responses in injured adult mammalian hearts. Given the high regenerative capacity of adult zebrafish hearts, we hypothesize that decellularized zebrafish cardiac ECM (zECM) made from normal or healing hearts can induce mammalian heart regeneration. Using zebrafish and mice as representative species of lower vertebrates and mammals, we show that a single administration of zECM, particularly the healing variety, enables cardiac functional recovery and regeneration of adult mouse heart tissues after acute myocardial infarction. zECM-treated groups exhibit proliferation of the remaining cardiomyocytes and multiple cardiac precursor cell populations and reactivation of ErbB2 expression in cardiomyocytes. Furthermore, zECM exhibits pro-proliferative and chemotactic effects on human cardiac precursor cell populations in vitro. These contribute to the structural preservation and correlate with significantly higher cardiac contractile function, notably less left ventricular dilatation, and substantially more elastic myocardium in zECM-treated hearts than control animals treated with saline or decellularized adult mouse cardiac ECM. Inhibition of ErbB2 activity abrogates beneficial effects of zECM administration, indicating the possible involvement of ErbB2 signaling in zECM-mediated regeneration. This study departs from conventional focuses on mammalian ECM and introduces a new approach for cardiac tissue regeneration.
Source: Science Advances. 18 Nov 2016: Vol. 2, no. 11, e1600844.
GRANT OF THE MONTH
PI: Jennifer Collinger and Michael Boninger
Title: Neural Control of Dexterous Manipulation and Translation to a Portable System
Description: This project allows for continued development of an intracortical brain-computer interface for restoring sophisticated upper limb function. We aim to advance BCI control from simple reach and grasp tasks to truly dynamic control where the BCI user can dynamically interact with objects in the environment. Neural activity recorded from motor cortex will be used to control a prosthetic limb while tactile feedback is provided via intracortical microsimulation through electrodes implanted in somatosensory cortex. An additional component of this project is to develop more portable recording technology to allow BCI to be used by an ambulatory population such as people with upper limb amputations. The University of Pittsburgh will partner with Blackrock Microsystems, Inc., to develop and pilot test this technology.
Source: DARPA
Term: August 16, 2016 – August 15, 18
Amount: $1,910,993
