April 2019 | VOL. 18, NO. 04| www.McGowan.pitt.edu
Potential Treatment for Chronic Pain Getting Closer

McGowan Institute for Regenerative Medicine affiliated faculty member Joseph Glorioso III, PhD, Co-founder, CODA Biotherapeutics, and Professor and Emeritus Chair, Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, is working on a gene therapy approach for treating chronic pain. CODA is developing engineered neurotransmitter receptors that are activated exclusively by orally bioavailable drugs to control the activity of hyperexcitable neurons responsible for chronic neuropathic pain. The gene encoding the receptor is delivered to dysfunctional neurons by proprietary viral vectors that are optimized for robust and targeted gene transfer. Standard neurosurgical procedures are used to administer these viral vectors directly to the neurons to be controlled. Once expressed, the engineered receptor can be activated by the drug to modulate neuronal activity. This enables the selective, tunable and reversible regulation of the receptor – and hence cellular activity – based on the dosing regimen of the drug. CODA is engineering receptors to have exquisite sensitivity for the pharmacological activator, which should dramatically limit off-target side effects that plague many pharmaceutical treatments.
As reported by Megan Molteni for Wired, in 2014 Dr. Glorioso co-founded CODA Biotherapeutics to develop a gene therapy approach for treating chronic pain. Based in South San Francisco, CODA has so far raised $19 million to engineer receptors in people’s sensory neurons that can be controlled by a small molecule drug. The idea is to use a virus that evolved in nature to infiltrate the hyperexcitable nerves responsible for many kinds of neuropathic pain—from arthritic joints to thrown-out backs and nerve damage caused by many cancer treatments. A one-time injection into the skin sends the virus into the nerve cells, delivering the instructions for making this tunable on/off switch. When a patient feels pain, they take the drug, which cuts the power to the neurons’ electrical activity and shuts down the perception of pain, with minimal body-wide side effects and risks of addiction. Dr. Glorioso expects it will be 18 to 24 months before an experimental therapy is ready to test in humans. CODA is starting first with types of pain so severe they’re basically untreatable, but the same approach could also be applied to other neurological conditions, including anxiety, Dr. Glorioso says.
More than 19 million Americans live with chronic neuropathic pain. Nearly 10 million Americans take opioids for long-term pain and 2.1 million are estimated by the National Institutes of Health to be addicted. Current pharmacological therapies such as opioids, anticonvulsants, tricyclic anti-depressants and channel inhibitors provide little relief while having significant side effects and a potential for addiction. Nerve stimulation therapies require batteries and wires that need maintenance and carry a risk of infections. Surgeries that destroy pathological neurons are a last resort and can have permanent adverse effects on peripheral nervous system function.
RESOURCES AT THE MCGOWAN INSTITUTE
May Histology Special
The Histology Stain of the Month: Toluidine Blue
Mast cells play a key role in the inflammatory process.
A mast cell is a myeloid derived cell and is part of the immune system. Mast cells contain distinctive granules rich in histamine and heparin. Although best known for their role in allergy and anaphylaxis, mast cells play an important protective role as well, being intimately involved in angiogenesis, defense against pathogens, and wound healing.
Toluidine blue is one of the most common stains for acid mucopolysaccharides and glycoaminoglycans, both of which are components of mast cells granules.
You’ll receive 25% off Toluidine blue Staining for the entire month of May when you mention this ad.
Contact the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or hartj5@upmc.edu or call 412-624-5265
As always, you will receive the highest quality histology work with the quickest turn-around time.
SCIENTIFIC ADVANCES
Separating Stem Cell Therapy Hope vs. Hype: More from Dr. Peter Rubin

In a follow-up to the recent McGowan Institute for Regenerative Medicine Scientific Retreat Panel Discussion, “Hope vs. Hype of Stem Cell Therapy,” J. Peter Rubin, MD, Chair of the Department of Plastic Surgery, the UPMC Endowed Professor of Plastic Surgery, Director of UPMC Wound Healing Services, and Professor of Bioengineering at the University of Pittsburgh, was a guest on Pittsburgh’s NPR News Station WESA program, The Confluence.
The host, Kevin Gavin, had Dr. Rubin further explore the good, bad, and the ugly of advertised, unproven stem cell treatments touted as curing all sorts of disease and maladies. As a faculty member at the McGowan Institute for Regenerative Medicine, a local hub of stem cell research, Dr. Rubin says he’s seen the benefits of stem cell therapy firsthand, including a number of injured soldiers he’s treated to correct facial deformities or treat pain after amputation.
But he warns some clinics have been allowed to prey upon medically vulnerable populations – costing some people thousands of dollars out of pocket – and that hyped-up advertising is harming legitimate stem cell research. Dr. Rubin advises patients to seek counsel from a physician that specializes in the area best qualified to treat a specific disease.
Listen to the entire interview here.
Physical Therapists Play Key Roles in Innovative Rehabilitation Technologies

Katherine Malmo recently reported for PT in Motion the importance of physical therapy in the success of future regenerative medicine therapies. As regenerative medicine helps the body restore biological function lost to age, disease, injury, or congenital abnormality, it can be accomplished with the help of medical devices and organs, biomaterials, and cellular therapies. These latter treatment options need support through focused, personalized physical therapy to be successful.
“It’s an exciting time,” says McGowan Institute for Regenerative Medicine faculty member Fabrisia Ambrosio, PT, PhD, MPT, Director of Rehabilitation for UPMC International and an Associate Professor in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh. “We’re seeing more research and even clinical trials evaluating the potential of stem cell transplantation to promote functional outcomes after stroke. There’s a lot of interest in understanding how rehabilitation—which of course is absolutely fundamental for functional recovery after stroke—can synergize with cellular therapy approaches.”
McGowan Institute affiliated faculty member Michael Boninger, MD, a professor and vice chair for research in the Department of Physical Medicine and Rehabilitation at the University of Pittsburgh School of Medicine, agrees that stem cells need to be trained and programmed through motion. For instance, he says, “If we really want to cure paralysis, we probably need to make sure stem cells understand the electrical signaling they need to do. So, there might need to be a brain-computer interface moving the arm as the person intended to move, so that the right connections are made.”
“For 30 or 40 years, people have been talking about using stem cells or other means of reconnecting a broken spinal cord,” Dr. Boninger says. “There has not been much success. One problem may be that the stem cells don’t know what to do when they’re implanted in a nonfunctioning cord.”
However, Dr. Boninger says, if the stem cells are given a “mechanical cue,” they’re much more likely to know how to differentiate themselves and become a functional stem cell that can turn into muscle or tissue.
“If we can decode the signals for movement from the brain and plant stem cells, then move the arm—possibly with an exoskeleton,” he continues, “all of these efforts together might actually create a better environment for the stem cells to work. That’s the regenerative medicine component. The pathways would be recreated by having a better understanding of which signals want to get through, then providing the milieu that enables them to work.”
This is another area in which physical therapy plays an important role.
“The hard work that physical therapists have been pushing people to do keeps being proven important,” Dr. Boninger says. “What physical therapists do has great scientific basis.”
Dr. Ambrosio also has been working to bridge the gap between PTs and rapidly advancing technology and science as co-director of the Alliance for Regenerative Rehabilitation Research and Training (AR3T). AR3T is a National Institutes of Health-funded resource center “supporting the expansion of scientific knowledge, expertise and methodologies across the domains of rehabilitation science and regenerative medicine.” It is a multi-institutional network of laboratories that includes those at the University of Pittsburgh, Stanford University, the Mayo Clinic, and the University of Texas at Austin.
With help from a $5 million grant from the National Institutes of Health, the organization provides funding for what its website calls the “development of novel lines of research” and education through webinars, sabbatical experiences, and research design consultations. Every year, it also hosts the Annual International Symposium on Regenerative Rehabilitation, with the goal of bringing scientists and clinicians together.
“For some time,” Dr. Ambrosio says, “basic scientists have used devices such as bioreactors to apply stimuli to stem cells at the laboratory bench, with the goal of promoting stem cell function. The next logical step is to evaluate how clinically-relevant mechanical, thermal, or electrical stimulation protocols, such as via exercise or modalities, might elicit similar stem cell benefits in vivo. The Symposium on Regenerative Rehabilitation allows basic scientists to gain increased exposure to cutting-edge rehabilitation technologies and approaches. It also exposes rehabilitation clinicians to advances in regenerative biology.
“This is important,” she continues, “because insights into stem cells and their role in tissue healing after injury or in the setting of disease has the potential to help inform the rational design of rehabilitation protocols. Our symposium is intended to be a mashup between the two fields.”
The 8th Annual International Symposium on Regenerative Rehabilitation will be held in Charlottesville, VA, October 24-26, 2019.
Abstract (The emerging relationship between regenerative medicine and physical therapeutics. Ambrosio F, Wolf SL, Delitto A, Fitzgerald GK, Badylak SF, Boninger ML, Russell AJ. Physical Therapy; 2010 Dec;90(12):1807-14.)
A Surgical Option to Treat Chronic Pancreatitis
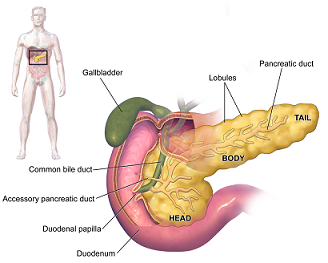
Total pancreatectomy with islet autotransplantation (TPIAT) is a surgical procedure used to treat severe complications of chronic pancreatitis (CP) or very high risk of pancreatic cancer while reducing the risk of severe diabetes mellitus.
In 1977, researchers at the University of Minnesota School of Medicine pioneered the first TPIAT for the treatment of CP. At that time, islet cell isolation techniques, which had been pursued to treat insulin-dependent diabetes via allotransplant, yielded variable results and raised uncertainty regarding the future efficacy of TPIAT. Since then, advances in isolation and purification have improved islet transplant outcomes, and the practice of TPIAT has expanded.
Today at UPMC, surgeons including McGowan Institute for Regenerative Medicine affiliated faculty member Abhinav Humar, MD, Clinical Director of the Thomas E. Starzl Transplantation Institute and the Chief, Division of Transplantation in the Department of Surgery at UPMC, performs about 10 of these surgeries a year.
“It’s a big surgery and you’re losing the pancreas,” says Dr. Humar.
In her report, Maria Simbra, MD, for KDKA/CBS Pittsburgh, says the problem with completely removing the pancreas is it has many important functions. It secretes enzymes important to digestion, and it makes insulin, a hormone important to process sugar and carbohydrates. The digestive enzymes are easily replaced, but without a pancreas, you would become diabetic.
In a day-long operation, the pancreas is taken out and sent to the lab. It is chemically treated and disintegrated.
The islet cells — the cells that produce insulin — are extracted and spun down into a small pellet. The pellet of cells is mixed with fluid, and the mixture is injected into the vein leading into the liver.
There, the islet cells make a new home. Patients take insulin until the cells get established.
“Ninety percent of patients plus get improvement of their pain symptoms,” says Dr. Humar. “It’s probably the most effective therapy we have for treatment of the pain.”
Some people do not get pain relief — related to trouble getting off the narcotics, or an issue with the pain nerves themselves.
As for the transplanted islet cells — one-third of the patients will have full function, one third will need some insulin, and another third will make no insulin of their own.
Generally, the procedure works better, the more functioning islet cells you have. The longer you have pancreatitis, the more the cells degrade, and the transplant is less likely to work.
“We’d like to try to see those patients earlier in their disease process because I think the likelihood of having a successful outcome is better in those types of situations,” says Dr. Humar.
Illustration: Pancreas. Wikipedia.
Abstract (Total pancreatectomy with islet autologous transplantation: the cure for chronic pancreatitis? Samuel J Kesseli, Kerrington A Smith, and Timothy B Gardner. Clinical and Translational Gastroenterology; 2015 Jan; 6(1): e73.)
How Immune Cells Help Tumors Escape Body’s Defenses

New research from the University of Pittsburgh School of Medicine and the UPMC Hillman Cancer Center sheds light on how tumors use the body’s regulators of immunity for their own benefit. Published in Nature Immunology, the findings could be used to develop the next generation of immune therapies to fight various cancers. McGowan Institute for Regenerative Medicine affiliated faculty member James Luketich, MD—Henry T. Bahnson Professor of Cardiothoracic Surgery, the Chairman, Department of Cardiothoracic Surgery, the Chief, Division of Thoracic and Foregut Surgery, the Director, Thoracic Surgical Oncology, the Co-Director, Lung Cancer Center, the Associate Director of Surgical Affairs, University of Pittsburgh Cancer Institute, and the Director, Mark Ravitch/Leon C. Hirsh Center for Minimally Invasive Surgery—is a co-author on the paper.
“While cancer immunotherapy drugs that block inhibitory cell surface molecules like PD1 have revolutionized cancer care, only about 20 percent of patients clear their tumors,” said senior author Dario Vignali, PhD, who holds the Frank Dixon Chair in Cancer Immunology, and is professor and vice chair of immunology at Pitt’s School of Medicine. “Our findings uncovered a previously unknown biological mechanism that reveals new therapeutic approaches to promote anti-tumor immunity.”
Dr. Vignali and his team focused on a group of immune cells called regulatory T cells (Tregs), which help maintain a delicate balance in the immune system. They do so by tamping down immune responses, keeping the system sensitive enough to catch threats, but not so sensitive that they result in autoimmunity – attacking normal cells. To achieve this control, Tregs release small proteins called cytokines that can have different effects on cells.
Previous research, including findings from Dr. Vignali’s lab, had shown that tumors cleverly take advantage of Tregs inside the tumor’s microenvironment to turn off killer T cells and evade the body’s immune defenses.
In the current study, first authors Deepali Sawant, PhD, a former post-doctoral fellow, and Hiroshi Yano, a current graduate student in Dr. Vignali’s lab, along with the rest of the team, set about trying to shed light on how Tregs turned off killer T cells using two inhibitory cytokines called interleukin-10 (IL-10) and interleukin-35 (IL-35).
Their first finding, in mouse and human tumors, was that populations of Tregs could either make IL-10 or IL-35 but, surprisingly, not both at the same time. “This was unexpected because we had anticipated that activated Tregs would use all tools available to them to suppress immune responses. Instead it seems they have to make a choice to secrete only one inhibitory cytokine,” noted Dr. Vignali, who also is co-leader of the Cancer Immunology and Immunotherapy Program at the UPMC Hillman Cancer Center.
The team then used a mouse model of cancer to show that for tumors to effectively suppress the immune system, they needed both types of Treg cells – those that secrete IL-10 and those that secrete IL-35.
“It’s like making a latte; you need both coffee and milk,” explained Vignali. “Without both, you won’t have a latte!”
Investigating further, they found that the cytokines, acting together, activated a protein called BLIMP1, which effectively disabled killer T cells by making them express a large variety of inhibitory cell surface molecules, such as PD1, LAG3, TIM3 and TIGIT, which suppresses their ability to detect and kill cancer cells.
This finding is important, says Dr. Vignali, because many current clinical trials are focused on immunotherapies that block just one or two such inhibitory proteins that would, in turn, activate killer T cells. But developing drugs that block IL-10 or IL-35 could have a much greater effect by preventing the expression of many inhibitory proteins at once.
The authors also note that since drugs targeting Tregs are already being tested in clinical trials, finding the precise mechanisms by which they work could provide clues to making more effective drugs, or help design better combinations with currently available immunotherapies to improve treatment success.
The study was funded by National Institutes of Health grants R01 CA203689, P01 AI108545, P30 CA047904 and S10 OD011925, and by Tizona Therapeutics. Dr. Vignali and study co-author Creg Workman, PhD, of Pitt, have submitted patents covering IL-35.
Illustration: UPMC/Hillman Cancer Center.
Abstract (Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Deepali V. Sawant, Hiroshi Yano, Maria Chikina, Qianxia Zhang, Mengting Liao, Chang Liu, Derrick J. Callahan, Zhe Sun, Tao Sun, Tracy Tabib, Arjun Pennathur, David B. Corry, James D. Luketich, Robert Lafyatis, Wei Chen, Amanda C. Poholek, Tullia C. Bruno, Creg J. Workman & Dario A. A. Vignali. Nature Immunology; online April 1, 2019.
Brain-Machine Interface Research Receives $12 Million from the NIH

Dr. Maria Simbra, Health Editor from Pittsburgh’s local CBS channel KDKA, recently visited the University of Pittsburgh’s Rehab Neural Engineering Lab for an update on the work being done with the sensorimotor microelectrode brain-machine interface and generating ‘touch’ sensations. She visited with Nathan Copeland who injured his spinal cord in a 2004 car accident, and he has been instrumental to the research in the lab.
Mr. Copeland has electrodes implanted in the parts of his brain that control movement and that process sensation. Not only is he able to pick up items just using his thoughts, he can feel them, too. With the research team, he has been traveling to neuroscience meetings to demonstrate. He gets questions like, “What does it feel like, would you use it at home, is it hard, do you like it?”
“What it feels like is complicated. I would definitely use it at home. It’s not hard. It’s pretty fun most of the time,” Mr. Copeland said.
The research team is made up of many people and includes Robert Gaunt, PhD, and McGowan Institute for Regenerative Medicine affiliated faculty member Michael Boninger, MD, Professor and UPMC Endowed Vice Chair for Research in the Department of Physical Medicine & Rehabilitation at the University of Pittsburgh, School of Medicine. The team is excited to be getting a $12-million grant from a new funding source, the National Institutes of Health. It allows for two more participants in Pittsburgh, and two at the University of Chicago.
“We plan on being able to accelerate the science by learning from all four of those individuals at the same time,” said Dr. Boninger. “So, when I press on something, I get immediate feedback to how hard I’m pressing, and it modulates how hard I press. Or when I’m trying to button something, I know where my fingers are without having to look at them. And those are the really critical things we need to work on in the next several years. Even being able to reach out and pick up an object and bring it to you, to scratch your face to itch an itch without having someone do it for you, these are the sorts of things that are really meaningful to people who can’t move at all.”
The team hopes two additional participants step up to take part in the new, expanded study. Of course, it is a lot of time, repetition, and hard work, and the robotic component is only available in the lab and not at home. But previous participants have appreciated being a pioneer.
Mr. Copeland travels to Japan in April for another neuroscience conference. In the meantime, he continues with the groundbreaking work in Pittsburgh.
Watch Dr. Simbra’s coverage of this program here.
Study Shows Key Factors in Sustaining Weight Loss Following Bariatric Surgery

In the years following bariatric surgery, a person’s overall eating behaviors and the amount of time spent watching television, playing video games and using a computer for recreation are a better indication of long-term weight loss success than specific weight control practices like counting calories.
Those findings by the University of Pittsburgh Graduate School of Public Health, published in the Annals of Surgery, also indicate that, despite the thorough psychological evaluation often required in preparation for bariatric surgery, mental health and eating habits prior to surgery were not useful in predicting which patients would struggle most with keeping the weight off after surgery. It is the largest long-term study to examine patient behaviors and characteristics associated with post-bariatric surgery weight regain. McGowan Institute for Regenerative Medicine affiliated faculty member Steven Belle, PhD, MScHyg, Professor in the Department of Epidemiology, Graduate School of Public Health at the University of Pittsburgh and a Co-Director in the Epidemiology Data Center in the Graduate School of Public Health, is a co-author on the study.
“Bariatric surgery is the most effective treatment for severe obesity. It results in sustained weight reduction and remission of diabetes and other health problems in the majority of patients,” said lead author Wendy King, PhD, associate professor in Pitt Public Health’s Department of Epidemiology. “However, as with all types of weight loss interventions, patients usually regain at least some of the weight they initially lose.”
Dr. King’s previous work, which quantified the tremendous variation among patients in the timing and amount of weight regained following maximum weight loss from bariatric surgery, highlighted the need for this study to identify behaviors related to weight regain and patients at risk for regaining the most weight. “This study is important for prevention and early intervention efforts,” she said.
Dr. King and her colleagues followed 1,278 adults who underwent Roux-en-Y gastric bypass surgery and had their weight measured an average of 8.3 times over a period that averaged 6.6 years. The participants were enrolled in the National Institutes of Health-funded Longitudinal Assessment of Bariatric Surgery-2 (LABS-2), a prospective, observational study of patients undergoing weight-loss surgery at one of 10 hospitals across the United States.
Reducing sedentary behavior; avoiding fast food; addressing problematic eating behaviors—including eating continuously, eating when full, loss of control and binge eating; and promoting self-weighing at least weekly were all behavioral targets the research team identified that patients should strive for and doctors should promote as part of post-surgical patient care.
In addition to evaluating behaviors, the scientists also identified patient characteristics related to higher weight regain. Younger patients and those with venous edema with ulcerations, which is a disease of the veins accompanied by sores on the skin; difficulty with daily physical function, such as bathing, dressing and walking; or depressive symptoms post-surgery gained more weight, suggesting they may warrant enhanced monitoring.
“This may sound like common sense,” said Dr. King. “But several behaviors and characteristics that clinicians hypothesized to matter were not related to weight regain. For example, while frequency of fast food consumption was associated with greater weight regain, frequency of meals or eating at restaurants were not.”
This study also demonstrated the difficulty of predicting outcomes of bariatric surgery based on pre-surgery status. For example, while depressive symptoms during the post-surgery period were related to weight regain, pre-surgery depressive symptoms were not. Likewise, pre-surgery eating behaviors were not useful for determining the risk of weight regain.
Anita Courcoulas, MD, senior author of the study and chief of minimally invasive bariatric surgery at UPMC, said the research builds on the team’s previous analyses indicating the importance of longer-term, close follow-up of patients to help maximize weight and health results following bariatric surgery.
“As clinicians, we know that weight maintenance is the most important, yet challenging, aspect of long-term post-bariatric surgery care,” she said. “Because we found that most individual patient characteristics at the time of surgery do not clearly identify those most at risk for poor weight loss maintenance after surgery, it is especially important that clinicians and programs engage with patients early and often after surgery about behaviors that can aid in limiting weight regain.”
Additional authors on this study are Amanda S. Hinerman, MPH, of Pitt; James E. Mitchell, MD, of the Neuropsychiatric Research Institute in Fargo, ND; and Kristine J. Steffen, PharmD, PhD, of North Dakota State University.
Abstract (Patient behaviors and characteristics related to weight regain after Roux-en-Y gastric bypass: a multicenter prospective cohort study. King, Wendy C., PhD; Belle, Steven H., PhD; Hinerman, Amanda S., MPH; Mitchell, James E., MD; Steffen, Kristine J., PharmD, PhD; Courcoulas, Anita P., MD, MPH. Annals of Surgery; online April 4, 2019.)
TEDx: Dr. Jelena Janjic, “When in Pain, Innovate!”

McGowan Institute for Regenerative Medicine affiliated faculty member Jelena Janjic, PhD, Associate Professor, School of Pharmacy, Graduate School of Pharmaceutical Sciences, Duquesne University, recently participated in Carnegie Mellon University’s TEDx program. In her talk entitled “When in Pain, Innovate,” Dr. Janjic challenged the current approach to pain management to designing new pain medicines and took her audience through innovations in nanotechnology that focus on a unique approach to remedy and diagnose pain. Watch her presentation here (starts around 4:06:20.)
Dr. Janjic pioneered nanotechnology development for chronic pain and her work is supported by the National Institutes of Health and the U.S. Air Force. The theranostic pain nanomedicine she designed is able to simultaneously image and modulate immune cells for therapeutic intervention in a number of inflammatory diseases including chronic pain. Her work on pain nanomedicine drew international and national attention. She won the Pittsburgh Business Times Innovator Award and was inducted into Duquesne University Research Hall of Fame in 2018. For the past 10 years, her focus was nanotechnology for imaging and drug delivery with two patents, more than 40 peer-reviewed publications and book chapters, and invited presentations at national and international meetings.
Dr. Janjic holds a pharmacy degree from Belgrade University, S.R. Yugoslavia, and a PhD in Medicinal Chemistry/Pharmaceutical Sciences from University of Pittsburgh School of Pharmacy. She completed post-doctoral training at Scripps Florida in drug discovery and Carnegie Mellon University in imaging nanotechnology. She is full time faculty at Duquesne University Pharmacy School since 2009. Dr. Janjic is the Founder and has served as a Co-Director of the Chronic Pain Research Consortium (CPRC) at Duquesne University since May 2011. She also serves as the ORISE Faculty Fellow and a Principal Scientist with the 59th Medical Wing U.S. Air Force, USAISR.
Bioengineering’s Role in Regenerative Medicine – From the Swanson School of Engineering

Starfish can repair injured arms and reptiles can regrow severed tails; from bacteria to humans, every species is capable of regeneration, albeit to variable extents. These functions help make species more resilient, but how can we apply the knowledge of these regenerative mechanisms to improve human health? The University of Pittsburgh Department of Bioengineering has been collaboratively working to address this question through research efforts in tissue engineering and regenerative medicine.
In 1981, upon the arrival of Dr. Thomas E. Starzl at the University of Pittsburgh Medical Center, Pittsburgh quickly became world-renowned in transplantation. This helped put Pittsburgh on the map in the medical community, eventually leading to increased interest in artificial organs and the establishment of the McGowan Center for Artificial Organ Development in 1992. This Center, which later became the McGowan Institute for Regenerative Medicine, continues to flourish and develop therapies that reestablish tissue and organ function impaired by disease, trauma, or congenital abnormalities.
The McGowan Institute of Regenerative Medicine received its current title in 2001 when the Center’s mission was expanded to include tissue engineering, adult-derived stem cell and wound healing research, and several other areas of investigation. “We could have carried on with medical device and artificial organ research, but we recognized that we wanted to address the problem – organ and tissue failure – and begin to develop a wider range of technologies,” said McGowan Institute director William Wagner, PhD, professor of surgery at Pitt.
Dr. Wagner, who joined the University in 1991, saw his own research begin to evolve beyond artificial organs. He studied blood coagulation from an engineering perspective, which turned out to be useful in understanding why artificial hearts were forming blood clots, but he later wanted to move beyond artificial hearts and look at ways to make tissue for cardiac repair. His research group currently focuses on developing cardiovascular technologies, with projects that address medical device biocompatibility and design, hypothesis-driven biomaterials development, tissue engineering, and targeted imaging.
Dr. Wagner believes that advancements in regenerative medicine require a collaborative effort in which bioengineering plays a large role. “An interdisciplinary approach is what makes the McGowan Institute thrive,” he said. “The problem we are trying to solve is what happens when a part of the body is no longer working. A clinical viewpoint is necessary, but medical training doesn’t give you an engineer’s perspective of design and manufacturing. You need a solid foot in both camps to make progress.”
This approach is well-suited for the University of Pittsburgh, which ranked fourth among U.S. universities in funding by the National Institutes of Health in FY 2018. These research opportunities are complemented by the world-renowned University of Pittsburgh Medical Center, which has helped to create a collaborative environment conducive to productive medical research.
“Institutes are created to bring people from different disciplines together to solve a common problem, and that is exactly what we are doing at the McGowan Institute,” said Dr. Wagner. “If you look in our laboratories, you could find a nursing professor, a pathology professor, a cardiac surgeon, or a bioengineer – it is a very diverse population. We have a wide range of research, and this center allows these collaborations to be built and supported.”
Among this wide range of researchers are 16 primary bioengineering faculty. From the design and development of novel artificial lung devices to the creation of an injectable gel technology that helps injured peripheral nerves repair and regenerate, bioengineering faculty have made an impact on biomedical translational research and innovation at Pitt.
“I think we are at a very exciting point where the commercialization of a lot of our inventions is not only possible but practical,” said Dr. Wagner. “Our ideas and the technology we have developed are more mature, and the investment community has a better idea of what we are trying to do.”
The translational research efforts at the McGowan Institute have led to 30 spinout companies derived from McGowan Institute affiliated faculty members. Bioengineering has contributed to the success of these efforts by offering the Center for Medical Innovation, which provides early-stage seed grants to projects, and the Coulter Translational Research Partners II Program, which provides funding and helps lead the projects to commercialization.
Beyond research, education is another priority of the McGowan Institute. Part of their mission is to educate and train scientists and engineers to pursue technologies related to regenerative medicine and train a generation of clinicians in the implementation of regenerative therapies. Bioengineering makes up a large part of the McGowan Institute graduate student population, which also includes surgical fellows and residents.
McGowan Institute affiliated faculty currently participate in three NIH training grants to support the educational efforts: The Biomechanics in Regenerative Medicine (BiRM) program, the Cardiovascular Bioengineering Training Program (CBTP), and the Cellular Approaches to Tissue Engineering and Regeneration (CATER) program. In addition, the McGowan Institute serves local, national, and international undergraduate students through the Regenerative Medicine Summer School, a hands-on, experiential learning program launched by McGowan Institute faculty member and bioengineering associate professor Bryan Brown, PhD, which aims to recruit students from underrepresented backgrounds, including those at universities without significant bioengineering and/or regenerative medicine programs. This educational program nicely complements the CampBioE effort by the Department of Bioengineering, which is a tissue engineering summer camp for middle and high school students.
“I consider the McGowan Institute to be a scaffold that brings together individuals interested in tissue engineering and regenerative medicine and facilitates their interactions,” said McGowan Institute faculty member Sanjeev Shroff, PhD, distinguished professor and Gerald E. McGinnis chair of bioengineering. “The symbiotic relationship between the McGowan Institute and the Department of Bioengineering greatly benefits our students and faculty, offering outstanding opportunities for training and research collaborations.”
All of these efforts have led the McGowan Institute of Regenerative Medicine to be one of the most prominent institutes of its kind. As this dynamic field evolves and grows, the Department of Bioengineering will continue to contribute to the diverse group of researchers at the McGowan Institute who pursue the development of innovative technology and new therapies for patients in need.
Source: University of Pittsburgh Swanson School of Engineering News Release
Vorp Lab Student Represented Pitt at the ACC Meeting of the Minds

Trevor Kickliter, a junior mechanical engineering student in the Swanson School of Engineering and a team member in the laboratory of McGowan Institute for Regenerative Medicine affiliated faculty member David Vorp, PhD, was selected as one of six undergraduate researchers to represent the University of Pittsburgh at the 2019 Atlantic Coast Conference (ACC) Meeting of the Minds Conference hosted by the University of Louisville, March 29-31, 2019. Mr. Kickliter presented his research on the use of adipose-derived mesenchymal stem cells (ADMSCs) as a promising alternative to traditional surgical therapy for an abdominal aortic aneurysm (AAA).
With a mortality rate of 90 percent and no sufficient strategy for early intervention, rupture of an AAA is one of the leading causes of death in the United States. The aorta is the largest blood vessel in the body, which runs from the heart, through the chest, and down to the abdomen. Due to its size, an AAA can lead to massive internal bleeding, which is typically fatal.
According to Mr. Kickliter, due to inadequate diagnostic markers, surgical intervention for this disease often fails to treat those in need of care while subjecting others to unnecessary risks. His work in the lab of Dr. Vorp, Associate Dean for Research and the John A. Swanson Professor of Bioengineering, addresses these shortcomings through the use of stem cell therapy.
“Our lab has previously investigated the use of adipose-derived mesenchymal stem cells in therapies for abdominal aortic aneurysm, but a method to effectively target ADMSCs to the aorta has yet to be developed or tested in large animals,” said Mr. Kickliter. “Since the use of ADMSCs as a therapeutic treatment seems promising, the primary goal of this study was to design and create a method for localizing ADMSCs in large animal aortas.”
The group implanted a diametric magnet into a harvested aorta loaded with ADMSCs that were treated with iron nanoparticles. The internal magnet was then able to draw the ADMSCs to the aortic adventitia – the outermost layer of connective tissue in the aorta.
“We looked at a cross-section of the treated aorta under fluorescent microscopy, and we found a significantly greater concentration of ADMSCs both on and around the aortic adventitia in the group where an internal magnet was used,” said Mr. Kickliter. “These results suggest that our method can be used to localize stem cell-based vascular therapies in other large animals, including humans.”
Each participating institution in the ACC Meeting of the Minds conference is allowed to select a total of six students to give three oral presentations and three poster presentations. Mr. Kickliter presented his research during the poster session.
“This is another outstanding recognition for Trevor, who continues to impress me with the quality of his research,” said Dr. Vorp. “This work introduces a novel way to localize delivery of stem cell therapy in large animals, and we hope that it will lead to improved treatment for abdominal aortic aneurysms.”
Illustration: Vascular Bioengineering Laboratory.
McGowan Institute Visits Local Elementary School

The McGowan Institute for Regenerative Medicine Educational Outreach Program takes its researchers many places. Recently, McKnight Elementary School Science in McCandless, PA, held a science night for its students and families.
At the event, McGowan Institute faculty member Bryan Brown, PhD (pictured top), Director of Educational Outreach and Associate Professor in the Department of Bioengineering with secondary appointments in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Clinical and Translational Science Institute at the University of Pittsburgh, conducted several regenerative medicine demonstrations to help explain “What is regenerative medicine?”
David Nascari (pictured bottom), an undergraduate student in the laboratory of McGowan Institute deputy director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the Institute, directed a motor learning activity for the students.
Their participation was very much appreciated by the school district, and a fun, science experience was had by all.
AWARDS AND RECOGNITION
Dr. Ipsita Banerjee Wins 2019 Faculty Diversity Award

McGowan Institute for Regenerative Medicine affiliated faculty member Ipsita Banerjee, PhD, associate professor of chemical and petroleum engineering at the University of Pittsburgh Swanson School of Engineering, is the recipient of the School’s 2019 Faculty Diversity Award.
“It would be an understatement to say that Ipsita earnestly strives each year to improve the academic environment fostering the success of under-represented minority students at the graduate, undergraduate and high school levels,” says McGowan Institute faculty member Steven Little, PhD, department chair of Chemical and Petroleum Engineering at the Swanson School.
The Faculty Diversity Award Committee cited Dr. Banerjee’s accomplishments as:
- Commitment to community engagement through active participation in INVESTING NOW program, as well as collaboration the Carnegie Science Center and REU programs;
- Leadership and mentorship for women in STEM, through participation in the Women in STEM Conferences and AlChE Women’s Initiative Committee (WIC);
- Recognized excellence in mentorship, including the 2016 Summer Research Internship (SRI) Faculty Mentor Award by PITT EXCEL program;
- Service to the Swanson School in the recruitment and retention of underrepresented students through various internal and external programs.
Beyond her work with organizations on campus, Dr. Banerjee devotes time and effort into programs like the Carnegie Science Center’s CanTEEN Career Exploration Program, sharing her experience with middle school girls and encouraging them to pursue an education in STEM. She has also been involved with the Women Student Networking conference, AlChE Women’s Initiatives Committee, and in panels for Women in Science and Medicine organized by UPMC.
In addition to the award, Dr. Banerjee will receive a $2,000 grant and induction into the Office of Diversity’s Champions for Diversity Honor Roll.
Dr. Banerjee’s mentees endorsed her nomination for this award because of her thoughtful support, encouragement and motivation. Her own professional success, they noted, makes her a valuable role model for other women and under-represented minorities in STEM.
“Being a Hispanic woman in the field of science and technology, it is sometimes hard to find examples of other women and/or minorities who have gone through the process of pursuing a career in academia with as much success as Dr. Banerjee has,” says Dr. Maria Jaramillo, a senior scientist at IVIVA Medical and the first graduate student to work with Dr. Banerjee at Pitt. She adds that Dr. Banerjee’s help and encouragement to network, collaborate with other scientists at Pitt and beyond, and present her research are among the things that have been most influential to her career. “These opportunities were instrumental for the continuance of my career in academia, and even today, several years after finishing my PhD under her supervision, Dr. Banerjee still provides great support.”
“Dr. Banerjee embodies the phrase ‘women empowering women,’” says Brittany Givens Rassoolkhani, a former PITT EXCEL Summer Research Intern who is now a PhD candidate at the University of Iowa. “Throughout my time working with her, it was apparent that she was both brilliant and dedicated. Most importantly, I was encouraged to also be dedicated and brilliant in my own work via the way she mentored myself and other students in the laboratory.”
Not only did Dr. Banerjee’s mentorship inspire her students to conduct their own research and find their professional paths, but it also inspired them to be better mentors themselves. Brittany Givens Rassolkhani notes that now she is also a mentor and never forgot the lessons Dr. Banerjee taught.
“Throughout this process, Dr. Banerjee has been instrumental in reminding me how important it is as a woman, particularly a woman of color, in the sciences and engineering to be cultivated in an environment that encourages women to be equal, if not better than, their male counterparts,” she says. “Dr. Banerjee never let my goals be big and scary, as I so often saw them; instead, in her eyes our goals as researchers were always achievable. I hope that when I become a professor and start my own laboratory, I am able to provide even half as much support to and faith in my students as I witnessed from Dr. Banerjee.”
Congratulations, Dr. Banerjee!
Illustration: University of Pittsburgh Department of Chemical and Petroleum Engineering.
Dr. Ivet Bahar Receives “Kadir Has” Outstanding Achievement Award in Turkey

The 15th Annual Kadir Has Awards were held on Friday, March 22, 2019, in Istanbul, Turkey. Member of the Science Academy, Ivet Bahar, PhD, received the “Kadir Has” Outstanding Achievement Award for her contributions to the development of theoretical and computational models for explaining the functional dynamics of biomolecular systems as well as mentoring and teaching a new wave of scientists. She was presented with the award by the Chairman of the Board of Trustees of Kadir Has University, Mr. Nuri Has, the Kadir Has Foundation President, Mr. Can Has, and the President of Kadir Has University, Dr. Sondan Durukanoglu Feyiz.
The Kadir Has Awards seek to recognize the outstanding accomplishments that Turkish scientists have made at the national and international level and to promote people and institutions that have contributed to the development of society.
Dr. Bahar is a Distinguished Professor, the John K. Vries Chair, and the Founding Chair in the Department of Computational and Systems Biology at the University of Pittsburgh’s School of Medicine. Dr. Bahar holds several other affiliations, including Director/Principal Investigator of three NIH-funded Centers, NIGMS Biomedical Technology and Research Center on Multiscale Modeling of Biological Systems (MMBioS), NIDA Center for Computational Drug Abuse Center (CDAR), and BD2K Center for Causal Modeling. She is an Associate Director at the University of Pittsburgh Drug Discovery Institute; the Founder and Executive Committee Member of Carnegie Mellon University/Pitt PhD Program in Computational Biology. Dr. Bahar is also an affiliated faculty member of the McGowan Institute for Regenerative Medicine.
Congratulations, Dr. Bahar!
Dr. Krzysztof Matyjaszewski Admitted to the Australian Academy of Science

McGowan Institute for Regenerative Medicine affiliated faculty member Krzysztof Matyjaszewski, PhD, J.C. Warner Professor of Natural Sciences at Carnegie Mellon University and served as the head of the Chemistry Department, has been admitted to the Australian Academy of Science as a Corresponding Member for his outstanding scientific contributions. His fundamental contributions have changed the face of free radical chemistry and polymer science.
In 1994, Dr. Matyjaszewski invented a method of polymerization—a chemical reaction to produce long chain-like molecules—using a copper catalyst. Called atom transfer radical polymerization (ATRP), Dr. Matyjaszewski’s method has spawned a prolific area of research and several industry developments, including the commercial production of specialty materials.
Corresponding Members of the Academy are eminent scientists not resident in Australia. They are elected based on scientific excellence, with consideration given to their connection to Australian science.
Congratulations, Dr. Matyjaszewski!
Important Upcoming Dates
Pittsburgh Life Sciences Week: “Discussion About Stem Cell Therapies”
May 14, 2019 at 10 AM, Bridgeside Point II
Click Here for more information
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#196 –– Dr. Edward Botchwey discusses his multidisciplinary research in microvascular remodeling, inflammation resolution, and host stem cells.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, Callahan DJ, Sun Z, Sun T, Tabib T, Pennathur A, Corry DB, Luketich JD, Lafyatis R, Chen W, Poholek AC, Bruno TC, Workman CJ, Vignali DAA
Title: Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion
Summary: Regulatory T cells (Treg cells) maintain host self-tolerance but are a major barrier to effective cancer immunotherapy. Treg cells subvert beneficial anti-tumor immunity by modulating inhibitory receptor expression on tumor-infiltrating lymphocytes (TILs); however, the underlying mediators and mechanisms have remained elusive. Here, we found that the cytokines IL-10 and IL-35 (Ebi3-IL-12α heterodimer) were divergently expressed by Treg cell subpopulations in the tumor microenvironment (TME) and cooperatively promoted intratumoral T cell exhaustion by modulating several inhibitory receptor expression and exhaustion-associated transcriptomic signature of CD8+ TILs. While expression of BLIMP1 (encoded by Prdm1) was a common target, IL-10 and IL-35 differentially affected effector T cell versus memory T cell fates, respectively, highlighting their differential, partially overlapping but non-redundant regulation of anti-tumor immunity. Our results reveal previously unappreciated cooperative roles for Treg cell-derived IL-10 and IL-35 in promoting BLIMP1-dependent exhaustion of CD8+ TILs that limits effective anti-tumor immunity.
Source: Nature Immunology. 2019 Apr 1. [Epub ahead of print]
GRANT OF THE MONTH
PI: Michael Boninger
Title: A Biomimetic Approach towards a Dexterous Neuroprosthesis
Description: Cervical spinal cord injury results in the loss of arm and hand function, which significantly limits independence and results in costs over the person’s lifespan. A brain-computer interface (BCI) can be used to bypass the injured tissue to enable control of a robotic arm and to provide somatosensory feedback. Two primary limitations of current state-of-the-art BCIs for arm and hand control are: (1) the inability to control the forces exerted by the prosthetic hand and (2) the lack of somatosensory feedback from the hand. In the proposed study, we seek to considerably improve dexterous control of prosthetic limbs by implementing decoding strategies that enable the user to not only control the movements of the arm and hand, but also the forces transmitted through the hand. We anticipate that our biomimetic approach to decoding will yield intuitive, dexterous control of the prosthetic hand. Tactile sensations will be conveyed to the user through intracortical microstimulation (ICMS) of somatosensory cortex. The spatiotemporal patterns of stimulation will be based on our basic scientific understanding of how tactile information is encoded in somatosensory cortex, which we expect will result in more natural and intuitive sensations. In order to achieve our goal of developing a dexterous neuroprosthesis, we have brought together a team with human BCI experience from the University of Pittsburgh along with the basic science expertise at both Pitt and the University of Chicago. We will collaborate with experts in implantable neurotechnology (Blackrock Microsystems) and robotics (The Biorobotics Institute) to ensure that the device hardware allows us to take a biomimetic approach for control and feedback with an eye toward clinical translation. A total of 4 participants will be tested in a multisite study to accomplish the following three specific aims. Aim 1: Evoke natural and intuitive tactile sensations through ICMS of somatosensory cortex. We expect that biomimetic ICMS will evoke sensations that more closely resemble everyday tactile sensations and intuitively convey information about contacted objects than does standard fixed-frequency ICMS. Aim 2: Derive kinematic and kinetic signals from motor cortex for hand control. We will assess the degree to which motor cortical neurons encode forces exerted on objects. Based on these observations, we will develop hybrid decoders that enable controlling both the movement and force using a synergy-based approach. Aim 3: Demonstrate improved arm and hand function with a biomimetic sensorimotor BCI that combines the sensory feedback developed in Aim 1 with the hybrid decoding developed in Aim 2. A battery of functional assessments will be used including novel metrics designed specifically for sensorimotor prosthetics along with well-established tests identified in the NIH Common Data Elements. We anticipate that subjects will substantially improve their dexterity using a biomimetic BCI as compared to non-biomimetic BCIs or BCIs without somatosensory feedback.
Source: NINDS
Term: September 30, 2018 – August 31, 2023
Amount: $1,330,521 year one
