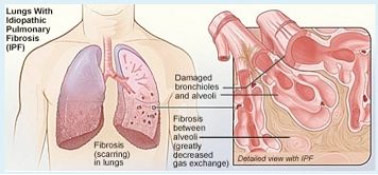
 When researchers at the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty members Charleen Chu, MD, PhD, the A. Julio Martinez Chair in Neuropathology and a Professor of Pathology in the Division of Neuropathology, Department of Pathology, University of Pittsburgh School of Medicine, and Mauricio Rojas, MD, Assistant Professor in the Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh—took a closer look at certain cells from the scarred lungs of patients with idiopathic pulmonary fibrosis (IPF), they were surprised by what they saw: many misshapen, bloated mitochondria. The unexpected observation led them to conduct a study that will be featured on the cover of the February issue of the Journal of Clinical Investigation, that could for the first time help explain why the risk of developing the deadly lung disease increases with age.
When researchers at the University of Pittsburgh School of Medicine—including McGowan Institute for Regenerative Medicine affiliated faculty members Charleen Chu, MD, PhD, the A. Julio Martinez Chair in Neuropathology and a Professor of Pathology in the Division of Neuropathology, Department of Pathology, University of Pittsburgh School of Medicine, and Mauricio Rojas, MD, Assistant Professor in the Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Pittsburgh—took a closer look at certain cells from the scarred lungs of patients with idiopathic pulmonary fibrosis (IPF), they were surprised by what they saw: many misshapen, bloated mitochondria. The unexpected observation led them to conduct a study that will be featured on the cover of the February issue of the Journal of Clinical Investigation, that could for the first time help explain why the risk of developing the deadly lung disease increases with age.
Older age is a well-known risk factor for IPF, a disease in which the lung tissue becomes progressively fibrotic, or scarred, leading to breathing difficulties and death within 3 to 5 years if a lung transplant isn’t possible, said senior investigator Ana L. Mora, MD, assistant professor in the Division of Pulmonary, Allergy and Critical Care Medicine and a member of the Heart, Lung, Blood and Vascular Medicine Institute (VMI) at Pitt. The cause of the disease is unknown, or “idiopathic.”
“Other chronic and progressive diseases we see with aging, such as Parkinson’s disease, have been recently associated with mitochondrial abnormalities, so we wondered if that was occurring in IPF,” she said. “It was a simple question, but it hadn’t been asked before, so we examined lung cells from patients with advanced IPF and healthy people. We were so surprised to see dramatic differences in the number, shape, and function of the mitochondria.”
After characterizing the oddities of the mitochondria, which provide energy for the cell, the team checked the levels of an enzyme called PTEN-induced putative kinase 1, or PINK1, that plays key roles in mitochondrial function and morphology, or shape. Experiments showed that impairment of mitochondria was associated with a reduction in PINK1 expression, and mice lacking PINK1 had dysfunctional, misshapen mitochondria in lung cells and were susceptible to developing lung fibrosis.
“We found also that low PINK1 is associated with increasing age and cellular stress,” Dr. Mora said. “This might help explain why older people are at greater risk for developing IPF, and it could mean developing drugs that can boost PINK1 levels or improve mitochondrial function will help treat IPF.”
“These findings are remarkable as they identify a similar disease pathway to that seen in other age-related brain diseases,” said Mark Gladwin, MD, professor and chief, Division of Pulmonary, Allergy and Critical Care Medicine, and VMI director, who is not on the research team. “This is the first study to find that the mitochondria themselves, the energy factories of our cells, are altered with lung fibrosis.”
In addition, the team hopes to find biomarkers to identify the disease in earlier stages as well as explore other factors that could increase susceptibility to IPF.
Illustration: National Heart Lung and Blood Institute, National Institutes of Health.
Read more…
UPMC/University of Pittsburgh Schools of the Health Sciences Media Relations News Release
Abstract (PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. Marta Bueno, Yen-Chun Lai, Yair Romero, Judith Brands, Claudette M. St. Croix, Christelle Kamga, Catherine Corey, Jose D. Herazo-Maya, John Sembrat, Janet S. Lee, Steve R. Duncan, Mauricio Rojas, Sruti Shiva1,7, Charleen T. Chu and Ana L. Mora. Journal of Clinical Investigation, published on-line 12/22/14.)
