After 40 years of blindness, a 58-year-old man can once again see images and moving objects, thanks to an injection of light-sensitive proteins into his retina.
The study, published in Nature Medicine, is the first successful clinical application in optogenetics, in which flashes of light are used to control gene expression and neuron firing. The technique is widely used in laboratories to probe neural circuitry and is being investigated as a potential treatment for pain, blindness, and brain disorders.
The clinical trial, run by the company GenSight Biologics based in Paris, enrolls people with retinitis pigmentosa (RP): a degenerative disease that kills off the eye’s photoreceptor cells, which are the first step in the visual pathway. In a healthy retina, photoreceptors detect light and send electrical signals to retinal ganglion cells (RGCs), which then transmit the signal to the brain. GenSight’s optogenetic therapy skips the damaged photoreceptor cells entirely by using a virus to deliver light-sensitive bacterial proteins into the RGCs, allowing them to detect images directly.
The researchers injected the virus into the eye of a man with RP, then waited four months for protein production by the RGCs to stabilize before testing his vision. McGowan Institute for Regenerative Medicine affiliated faculty member José-Alain Sahel, MD, Chair and Distinguished Professor of the Department of Ophthalmology at the University of Pittsburgh School of Medicine, Director of the UPMC Eye Center, the Eye and Ear Foundation Endowed Chair of Ophthalmology, and leader of the study, says that one of the challenges was regulating the amount and type of light entering the eye, because a healthy retina uses a variety of cells and light-sensitive proteins to see a wide range of light. “No protein can replicate what the system can do,” he says. So, the researchers engineered a set of goggles that captured the visual information around the man and optimized it for detection by the bacterial proteins.
Using a camera, the goggles analyze changes in contrast and brightness and convert them in real time into what Dr. Sahel describes as a ‘starry sky’ of amber-colored dots. When the light from these dots enters a person’s eye, it activates the proteins and causes the RGCs to send a signal to the brain, which then resolves these patterns into an image.
The trial participant had to train with the goggles for several months before his brain adjusted to interpret the dots correctly. “He was like an experimentalist, a scientist trying to understand what he was seeing and make sense of it,” Dr. Sahel says. Eventually, he was able to make out high-contrast images, including objects on a table and the white stripes in a crosswalk. When the researchers recorded his brain activity, they found that his visual cortex reacted to the image in the same way as it would have if he had normal sight.
The man still can’t see without the goggles, but Dr. Sahel says that he wears them for several hours per day and that his vision has continued to improve in the two years since his injection. Six other people were injected with the same light-sensitive proteins last year, but the COVID-19 pandemic delayed their training with the goggles. Dr. Sahel expects to have their results within about a year.
Safe and permanent
“It’s a big step for the field,” says John Flannery, PhD, a neurobiologist at the University of California, Berkeley. “The most important thing is that it seems to be safe and permanent, which is really encouraging.” Because the retina contains around 100 times more photoreceptors than do RGCs, the resolution of images detected by RGCs will never be as good as natural vision. But Dr. Flannery says it is exciting that the brain can interpret images accurately.
Others say that more research is needed. “It’s interesting, but it’s an N of 1,” says Sheila Nirenberg, PhD, a neuroscientist at Weill Cornell Medical College in New York City. She says she looks forward to seeing whether the other people in the trial, including some who were injected with higher doses of the protein, have similar results.
GenSight is one of several companies developing optogenetics as a treatment for RP and other disorders of the retina. In March, Dr. Nirenberg’s company Bionic Sight announced that four of the five people with RP it had treated with a similar optogenetic therapy and a virtual-reality headset had recovered some level of vision, although the full trial results have not yet been published. And Swiss pharma giant Novartis is developing a therapy based on a different protein that is so light sensitive that goggles might not be needed. That therapy has not yet entered clinical trials.
Karl Deisseroth, MD, PhD, a neuroscientist at Stanford University in California who co-developed optogenetics as a lab technique, says the study is important because it is the first time that the technique’s effects have been shown in people. “It will be interesting to try this with more light-sensitive opsins” that might not require goggles, he says. But he expects optogenetics to be most useful as a research tool that leads to therapies, rather than a therapy itself. “What we hope to see even more of is optogenetics-guided human and clinical studies,” he says.
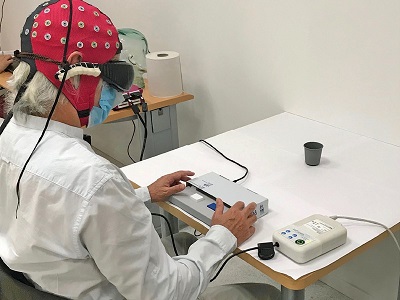
Illustration: Experimental setup for studying behavioral responses and brain activity. A visual detection task from the study. The man is asked to determine the presence or absence of a black cup on the white desk. Credit: J.-A. Sahel et al./Nature Medicine.
Read more…
Abstract (Partial recovery of visual function in a blind patient after optogenetic therapy.
José-Alain Sahel, Elise Boulanger-Scemama, Chloé Pagot, Angelo Arleo, Francesco Galluppi, Joseph N. Martel, Simona Degli Esposti, Alexandre Delaux, Jean-Baptiste de Saint Aubert, Caroline de Montleau, Emmanuel Gutman, Isabelle Audo, Jens Duebel, Serge Picaud, Deniz Dalkara, Laure Blouin, Magali Taiel & Botond Roska. Nature Medicine, May 24, 2021.)
