McGowan Institute for Regenerative Medicine affiliated faculty member Thomas Rando, MD, PhD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, and California and Florida researchers recently developed a two-pronged method that makes mouse leg muscles regrow better. Their research was recently published in Communications Biology.
As reported by Mandy Erickson for Scope, after a traumatic injury to arms and legs, surgeons will first repair bone and nerves, because without them, you can’t move your limbs. They worry about the muscle later: Muscle can regenerate to some degree, and even with diminished muscle, you can still move.
“Muscle is lower down on the totem pole,” said Ngan Huang, PhD, an assistant professor in cardiothoracic surgery and a principal investigator at Veterans Affairs Palo Alto Health Care System. In her work at the VA, Dr. Huang has seen how muscle injuries from war trauma can cripple veterans: “The patients still have some muscle — they can use the limb — but it’s much weaker.”
The only option currently available is to transplant muscle from another part of a patient’s body. It doesn’t always work, but even when it does, it weakens the muscle that acted as a donor. Dr. Huang and her team wondered: What if we are able to give injured muscle a boost?
The recipe for enticing muscle to regenerate involves laying strips of collagen — a fibrous protein found in the skin and connective tissues, among other areas — in neat parallel rows in a petri dish. Then, researchers add endothelial cells, a type of cell found on the insides of blood vessels. After allowing the cells to multiply for nine days, researchers implanted the endothelium-enriched scaffolding into the injured muscle.
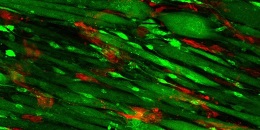
The photo shows the scaffolding on day nine; endothelial cells appear red, and muscle cells appear green.
For comparison, the researchers also implanted scaffolding without endothelial cells as well as scaffolding that wasn’t arranged in rows. The muscles with parallel scaffolding plus endothelial cells functioned far better than the control muscles: They contracted in a more synchronized manner when they were stimulated electrically, and they contained more blood vessels; the muscle cells were also longer.
“We didn’t think the combination of aligned scaffolding and endothelial cells would be so effective,” Dr. Huang said, but she has a clue why it worked: The neat rows coaxed the muscle progenitor cells to form parallel bundles of muscle fibers, the way they are normally organized in the body.
“Regeneration is a complex process,” she said. “The scaffolding orients the cells; it provides spatial cues. The endothelial cells are promoting integration with the vessels of the host. Both are equally important for muscle regeneration to happen in an effective way.”
The researchers plan to continue studying the technique and, if it appears successful, eventually try it out in patients.
Illustration: Scaffolding on day nine; endothelial cells appear red, and muscle cells appear green. Courtesy of Karina Nakayama.
Read more…
Stanford University/Scope News Release
Abstract (Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Karina H. Nakayama, Marco Quarta, Patrick Paine, Cynthia Alcazar, Ioannis Karakikes, Victor Garcia, Oscar J. Abilez, Nicholas S. Calvo, Chelsey S. Simmons, Thomas A. Rando and Ngan F. Huang. Communications Biology 2, Article number: 170 (2019).)
