Can nanomedicine play a role in the future of pain treatment? Several answers to this question were presented during the American Pain Society Scientific Summit in Anaheim, California, earlier this year. McGowan Institute for Regenerative Medicine affiliated faculty member Jelena Janjic, PhD, Associate Professor of Pharmaceutics in the Graduate School of Pharmaceutical Sciences and Mylan School of Pharmacy at Duquesne University and the Founder and Co-Director of the Chronic Pain Research Consortium at Duquesne, provided one solution through her work on targeted nanomedicine for chronic pain treatment.
As reported by Ashley Cowie for the Pain Research Form, Dr. Janjic said that nanomedicine promises to improve drug delivery for pain treatment—
- by decreasing the amount of drug needed to alleviate pain
- by enabling treatment adjustment as the patient recovers
- by offering the potential to release drugs upon disease flare-ups, and
- by targeting drugs only to specific tissues.
Dr. Janjic has designed a new Theranostic Analgesic Regenerative Gel-Emulsion Technology (TARGET) platform for localized delivery of an anti-inflammatory drug to the site of injury. TARGET works by incorporating a nanoemulsion containing a drug into a gel, which helps promote extended drug release at the site of injury. The newly developed TARGET platform is being evaluated for safety, feasibility, and efficacy in experimental pain models at the research facilities of the 59th Medical Wing of the United States Air Force.
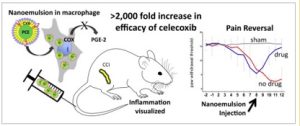
Macrophages associated with disease typically derive from blood monocytes and have two phenotypes: M1 macrophages are pro-inflammatory, while M2 macrophages are anti-inflammatory. Macrophages can switch between these phenotypes based on the cytokine signals they receive. Chronic inflammatory and neuropathic pain is perpetuated by M1 macrophages that express the enzyme cyclooxygenase-2 (COX-2). COX-2 signaling leads to elevated levels of prostaglandin E2 (PGE2), which is a physiologically active lipid linked to neuropathic pain through its effects on neuronal activity, cytokine production, and neuronal gene expression. Nonsteroidal anti-inflammatory drugs (NSAIDs) that inhibit COX-2 block production of PGE2. Thus, Dr. Janjic developed near infrared fluorescence-labeled (NIRF) theranostic nanoemulsions containing the COX-2-selective inhibitor celecoxib to target macrophages. In this instance, NIRF is a fluorescence technique that allows for visualization of inflammation.
As part of this collaborative project, Dr. Janjic and McGowan Institute affiliated faculty member John Pollock, PhD, Professor of Biological Science at Duquesne University, delivered the nanoemulsion via tail vein injection into rats eight days after they underwent chronic constriction injury (CCI), a model of neuropathic pain. These animals displayed significantly less mechanical hypersensitivity compared to rats that received a nanoemulsion containing no drug. Measurement of inflammation using NIRF labeling of macrophages that had engulfed the nanoemulsions without the drug revealed heightened fluorescence over the injured sciatic nerve, which was significantly decreased in animals that received the drug-containing nanoemulsion. This suggested that the macrophages containing celecoxib were most likely not of the inflammatory phenotype and did not migrate to the site of injury.
Next, the researchers studied the effects of the nanoemulsions on the number of infiltrating monocyte-derived macrophages, COX-2 expression, and PGE2 expression in the injured sciatic nerve using immunofluorescence. Treatment with celecoxib-containing nanoemulsions significantly reduced all three, compared to treatment with nanoemulsions without the drug.
In summary, nanoemulsion therapy significantly reduced inflammation at the site of injury, which coincided with decreased pain hypersensitivity. This holds promise for future therapies to utilize nanomedicine, allowing for low-dose, targeted drug delivery that avoids damage to healthy tissues.
Illustration: Journal of Neuroimmunology.
Read more…
Abstract (Low-dose NSAIDs reduce pain via macrophage targeted nanoemulsion delivery to neuroinflammation of the sciatic nerve in rat. Janjic JM, Vasudeva K, Saleem M, Stevens A, Liu L, Patel S, Pollock JA. Journal of Neuroimmunology; 2018 May 15;318:72-79.)
