October 2015 | VOL. 14, NO. 10 | www.McGowan.pitt.edu
Lab-Grown 3-D Intestine Regenerates Gut Lining in Pre-Clinical Trial
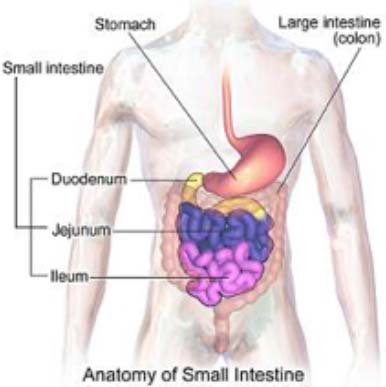 Working with gut stem cells from humans and mice, scientists from the Johns Hopkins Children’s Center and the University of Pittsburgh have successfully grown healthy intestine atop a 3-D scaffold made of a substance used in surgical sutures. In a further step that takes their work well beyond proof of concept, McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, Pitt graduate students Jenna Dziki and Timothy Keane, and researchers report their laboratory-created intestine successfully regenerated gut tissue in the colons of pre-clinical animals with missing gut lining.
Working with gut stem cells from humans and mice, scientists from the Johns Hopkins Children’s Center and the University of Pittsburgh have successfully grown healthy intestine atop a 3-D scaffold made of a substance used in surgical sutures. In a further step that takes their work well beyond proof of concept, McGowan Institute for Regenerative Medicine deputy director Stephen Badylak, DVM, PhD, MD, Pitt graduate students Jenna Dziki and Timothy Keane, and researchers report their laboratory-created intestine successfully regenerated gut tissue in the colons of pre-clinical animals with missing gut lining.
The experiments, described ahead of print in the journal Regenerative Medicine, bring researchers closer to creating an implantable intestine as replacement therapy for a range of devastating disorders – including infections, cancer, and trauma – that result in loss or death of gut tissue. Chief among them is a condition that affects 12 percent of premature newborns, called necrotizing enterocolitis (NEC), which is marked by the rapid death of intestinal cells and permanent loss of intestinal tissue.
The tube-shaped scaffold, designed several years ago in collaboration with Cornell University researchers and composed of biodegradable material similar to that used in surgical sutures, was a big first step on the quest to develop an implantable replacement intestine. But the new work pushes that effort further because it shows how stem cells, when mixed with immune and connective tissue cells, can grow into normal gut tissue around the scaffold and function inside a living mammal.
Researchers caution that a fully functioning replacement intestine for humans is far off, but they say their results have laid the critical groundwork to do so.
“Our experiments show that the architecture and function of our lab-made intestine strikingly resemble those of the healthy human gut, giving us real hope that our model could be used as the backbone for replacement intestine,” says principal investigator David Hackam, MD, PhD, the Johns Hopkins Children’s Center’s surgeon-in-chief, who initiated and conducted most of the work at the University of Pittsburgh.
In an initial set of experiments reminiscent of a peanut butter-and-jelly sandwich technique, researchers took stem cells from the colons of babies undergoing intestinal surgeries and from mice, then added immune cells called macrophages, the body’s scavengers that seek out and engulf debris along with foreign and diseased cells. To this mix, they added cells called fibroblasts, whose function is to form collagen and other connective substances that bind tissues and organs together. The idea, the scientists say, was to create a mixture that closely mimics the natural composition of the gut.
“Intestinal cells do not grow and develop in a vacuum, so we sought to recreate the richness of the human gut, Dr. Hackam says.”We took the PB & J approach, adding a layer of stem cells on top of the scaffold, then topping them with a mixture of immune and collagen cells.”
Adding these components, they report, enhanced the growth of intestinal stem cells and differentiation into various mature cell types critical to the function of a healthy intestine. In comparison, stem cells grown in isolation without immune and connective cells in the mix grew more slowly and failed to differentiate well into multiple cell types.
In a final step, the investigators implanted pieces of the newly created intestine – about 1.6 inches in length – into the lower portion of animal colons lacking parts of their intestinal lining. For 2 months, the animals underwent periodic colonoscopies and intestinal biopsies. Strikingly, the guts of the animals with implanted intestines healed completely within 8 weeks. By contrast, the animals that didn’t get intestinal implants experienced continued inflammation and scarring of their guts.
“Our results move research beyond the proof-of-concept realm,” says study author Dr. Badylak, professor of surgery at the University of Pittsburgh. “These results demonstrate that a mixture of synthetic and natural tissue can spur the formation of new gut cells and function well in living organisms despite the presence of naturally occurring inflammation and microbes found in the living gut.”
Illustration: Wikipedia.
RESOURCES AT THE MCGOWAN INSTITUTE
November Special at the Histology Lab
Safranin O Stain
This stain is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections, and may be used for frozen sections as well. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained tissue around the scaffold and function inside a living mammal.
You’ll receive 25% off Safranin O Staining for the entire month of November when you mention this ad.
Contact Lori at the McGowan Core Histology Lab and ask about our staining specials. Email perezl@upmc.edu or call 412-624-5265. As always, you will receive the highest quality histology in the quickest turn-around time.
Isolating Pericytes and RNA: A Flow Cytometry Protocol Developed
Efforts at the Flow Cytometry Lab are continuing to successfully support the various research efforts of McGowan Institute for Regenerative Medicine and University of Pittsburgh scientists. Flow cytometry is used to perform several procedures including cell counting, cell sorting, detection of biomarkers, and protein engineering.
Latha Satish, MSc, MPhil, PhD, is a McGowan Institute for Regenerative Medicine affiliated faculty member and a research assistant professor in the Department of Plastic Surgery at the University of Pittsburgh. She recently utilized the services of the Flow Cytometry Lab personnel and shares her experience here:
I had the opportunity to recently work with Ms. Lynda Guzik on isolating pericytes from the stromal vascular fraction (SVF) of the abdominal subcutaneous adipose (fat) tissue derived from healthy and diabetic patient populations. Previous studies have identified a pericyte population in these tissues, but these studies have not attempted to isolate RNA directly from these tissues or to expand them in culture. We embarked on this mission with guidance and advice from Ms. Guzik.
The goal was to sort pericytes directly from the SVF pellet for CD146+/CD73+/ CD105+/CD56-/CD34-/CD45- using the BD FACS AriaII. The purified pericytes would then be divided into two fractions for either immediate RNA extraction or in vitro growth and expansion in pericyte growth medium prior to RNA extraction. The first few attempts to achieve this goal failed as we could not achieve good cell viability.
With Ms. Guzik’s help, we identified that the bulk SVF sample was very clumpy and the cell numbers during staining were higher than expected. To solve this problem, Ms. Guzik advised us to pass the cells through a cell strainer and then count the cells to adjust the concentration appropriately. Following this we were able to obtain a good yield of viable pericytes.
Since pericytes comprise only 1% of the total cell population in the SVF fraction, the amount of RNA that we obtained was miniscule. But the purity and the quality of RNA was excellent due to Ms. Guzik’s excellent technical skills operating the instrument to obtain the best possible cell yield and purity. RNA isolation was achieved using 15,000-20,000 sorted pericytes using Prelude® Direct Lysis Module (NuGen Technologies Inc. San Carlos, CA). Extracted RNA was amplified to cDNA (Ovation® Pico WTA system V2, NuGen Technologies) and used for qRT-PCR analysis. Using this approach we were able to obtain ~350-425 ng/µl of cDNA allowing differential gene expression analysis without exposing the cells to in vitro culture conditions. [Figure 1: Sorting of pericytes from non-diabetic (A & B) and diabetic (C & D) SVF pellet by fluorescence-activated cell sorting (FACS). Cells were sorted for CD45- CD56- CD34- CD146+ CD105+ CD73+expression. Shown is a representative image of sorts performed on SVF pellets obtained from two subjects, non-diabetic (top) and diabetic (bottom).]
We also allowed the FACS-sorted pericytes to grow and expand in vitro using pericyte growth medium. After 14 days in culture, RNA was extracted and subjected to real time RT-PCR and compared to the RT-PCR results from cultured vs freshly isolated cells. We determined that RNA isolated using both methods did not show any significant difference in their gene expression patterns. [Figure 2: Real time RT-PCR showing the expression of select candidate genes comparing RNA isolated from non-diabetic and diabetic pericytes sorted from SVF pellet (A) and from RNA isolated from sorted non-diabetic and diabetic pericytes expanded in cultures (B). Relative quantification of gene expression was calculated by comparing δ Ct values between non-diabetic and diabetic sorted pericytes. GAPDH was used as internal control. Data was obtained from two each of non-diabetic and diabetic subjects performed in triplicate. Statistical significance was determined by student’s test (**p<0.001; *p<0.05) n=2).]
With the help of Ms. Guzik we have standardized our flow protocol to obtain the highest purity of pericytes.
Flow cytometry has numerous applications in science, including those relevant to healthcare. The technology has been widely used in the diagnosis of health conditions, particularly diseases of the blood such as leukemia, although it is also commonly used in a wide variety of fields from clinical practice, clinical trials, and basic research.
Other fields utilizing this technology include molecular biology, immunology, pathology, as well as marine science and plant biology. In medicine, flow cytometry is a vital laboratory process used in transplantation, oncology, hematology, genetics, and prenatal diagnosis. Flow cytometry can also be used in the field of protein engineering, to help identify cell surface protein variants.
Contact Lynda Guzik, the Flow Cytometry Lab Manager, for more information on how the Lab’s resources can help you: 412-648-8660 or guzilj@upmc.edu.
UPCOMING EVENTS
Save the Date: 9th Symposium on Biologic Scaffolds for Regenerative Medicine
The 9th Symposium on Biologic Scaffolds for Regenerative Medicine will be held at the Silverado Resort in Napa, California on April 28th – 30th, 2016.
 This symposium represents an opportunity to advance the use of biologic scaffolds for regenerative medicine and all general surgery applications. Topics will include the basic science of scaffold remodeling from the molecular level through the macroscopic and clinical level. Approximately 50% of the presentations will involve the clinical perspective in the form of formal studies, anecdotal reports, and surgeon reviews. Speakers will provide an objective opinion of the pros and cons of the use of biologic scaffold materials. It is not the intent of this symposium to discuss only the beneficial aspects of biologic scaffolds or particular products, but also to identify problems, develop strategies for solving these problems, and hopefully initiate collaborations among basic scientists, clinicians, and industry in attendance at the meeting.
This symposium represents an opportunity to advance the use of biologic scaffolds for regenerative medicine and all general surgery applications. Topics will include the basic science of scaffold remodeling from the molecular level through the macroscopic and clinical level. Approximately 50% of the presentations will involve the clinical perspective in the form of formal studies, anecdotal reports, and surgeon reviews. Speakers will provide an objective opinion of the pros and cons of the use of biologic scaffold materials. It is not the intent of this symposium to discuss only the beneficial aspects of biologic scaffolds or particular products, but also to identify problems, develop strategies for solving these problems, and hopefully initiate collaborations among basic scientists, clinicians, and industry in attendance at the meeting.
This symposium is designed to advance the use of biologic scaffolds for regenerative medicine and all general surgery applications via a series of objective presentations describing the potential benefits and risks associated with the use of such materials, factors that affect performance, and the clinical applications that may benefit most from their use. Topics range from the most basic science of scaffold remodeling at the molecular level through the preclinical and clinical level. It is not the intent of this symposium to discuss only the beneficial aspects of biologic scaffolds, but just as importantly to identify the problem areas, develop strategies for solving these problems, and hopefully initiate collaborations among basic scientists and clinicians in attendance at the meeting. Our feedback from previous symposia (this is a bi-annual event) consistently identifies the equal mix of clinicians and basic scientists as the most beneficial and rewarding aspect of the meeting;
Although all topic areas are considered and will be represented, the 2016 symposium will definitely include the following:
- An in depth account of the most recent findings regarding the mechanisms by which biologic scaffolds facilitate constructive remodeling of tissues; including body wall (skeletal muscle), cardiovascular, reconstructive surgical applications such as breast and pelvic floor, and whole organs such as liver, lung and heart.
- Identification and discussion of manufacturing issues such as tissue source, decellularization methods, and sterilization decisions that affect the quality and performance of biologic scaffolds for surgical applications.
- A review of clinical experiences, especially general surgery, orthopedic and trauma related challenges, neurologic applications, gastrointestinal applications, and cardiovascular applications.
- Identification and discussion of the effect of the host innate immune response upon scaffold remodeling and clinical outcome.
List of invited (and accepted) speakers to date: Robert M. Nerem, PhD (Georgia Institute of Technology), Arnold I. Caplan, PhD (Case Western Reserve University), Jeffrey M. Davidson, Ph.D. (Vanderbilt University), Cyrus Ghajar, PhD (Fred Hutchinson Cancer Research Center), Jeffrey A. Hubbell, PhD (Swiss Federal Institute of Technology, EPFL), Kristen Jones, MD (University of Minnesota), C. James Kirkpatrick MD PhD DSc FRCPath (Johannes Gutenberg University), Robert G. Martindale, MD, PhD (Oregon Health & Science University), Charles D. Mills, PhD (BioMedical Consultants), Laura E Niklason PhD, MD (Yale University), Frederick J. Schoen, MD, PhD (Harvard University), Allan S. Stewart, MD (Mount Sinai Hospital NYC), and Nadia Rosenthal, PhD (Monash University, Australia).
SCIENTIFIC ADVANCES
Pitt Researchers Are Working to Mass-Produce Stem Cells
 Human stem cells hold great promise for medicine. They can be used therapeutically, they can help model disease, and they can be used to help discover new drugs. But it is very difficult to culture enough cells to meet the demand.
Human stem cells hold great promise for medicine. They can be used therapeutically, they can help model disease, and they can be used to help discover new drugs. But it is very difficult to culture enough cells to meet the demand.
McGowan Institute for Regenerative Medicine affiliated faculty members Ipsita Banerjee, PhD, associate professor of chemical and petroleum engineering and of bioengineering within the University of Pittsburgh’s Swanson School of Engineering, and co-investigator Prashant Kumta, PhD, professor of bioengineering in the Swanson School, recently received a $300,000 grant from the National Science Foundation to explore an idea they have that would allow the manufacture of human pluripotent stem cells—these are the ones that can become virtually any tissue—on an industrial scale.
At present, Dr. Banerjee says, culturing stem cells is a delicate proposition, with only a 30 percent chance of survival. Current methods are limited by the low viability of initial cell-seeding populations and a tendency for the cells to be heterogeneous, a problem when uniformity is required.
Drs. Banerjee and Kumta think that by creating a biomimetic hydrogel encapsulation system to mimic the cell-to-cell interaction without which stem cells die, they’ll be able to overcome these obstacles.
“It’s kind of a simple idea, but it hasn’t been done,” Dr. Banerjee says. “I thought someone must have done it, at least with other kinds of cells, but no one has.”
The capsules she and Dr. Kumta plan to create will use an as-yet-unidentified peptide to convince individual cells they’re in the company of others, enhancing survival. “[A cell] will think that it is in contact with another cell and will not activate the cell-death pathway,” Dr. Banerjee says.
If they find the right peptide and their theories about stem cell behavior and viability are confirmed, Drs. Banerjee and Kumta are hopeful that their method of stem-cell cultivation will allow labs around the world to generate enough stem cells to meet demand.
“Our method is cheap and simple,” Dr. Banerjee concludes. “If we’re right, just about anyone in any lab will be able to reproduce it.”
Digestible Batteries Needed to Power Electronic Pills
 Imagine a “smart pill” that can sense problems in your intestines and actively release the appropriate drugs. We have the biological understanding to create such a device, but we’re still searching for electronic materials (like batteries and circuits) that pose no risk if they get stuck in our bodies. Published recently, McGowan Institute for Regenerative Medicine affiliated faculty member Christopher Bettinger, PhD, Assistant Professor in the Departments of Biomedical Engineering and of Materials Science and Engineering at Carnegie Mellon University, presents a vision for creating safe, consumable electronics, such as those powered by the charged ions within our digestive tracts.
Imagine a “smart pill” that can sense problems in your intestines and actively release the appropriate drugs. We have the biological understanding to create such a device, but we’re still searching for electronic materials (like batteries and circuits) that pose no risk if they get stuck in our bodies. Published recently, McGowan Institute for Regenerative Medicine affiliated faculty member Christopher Bettinger, PhD, Assistant Professor in the Departments of Biomedical Engineering and of Materials Science and Engineering at Carnegie Mellon University, presents a vision for creating safe, consumable electronics, such as those powered by the charged ions within our digestive tracts.
Edible electronic medical devices are not a new idea. Since the 1970s, researchers have been asking people to swallow prototypes that measure temperature and other biomarkers. Currently, there are ingestible cameras for gastrointestinal surgeries as well as sensors attached to medications used to study how drugs are broken down in the body.
“The primary risk is the intrinsic toxicity of these materials, for example, if the battery gets mechanically lodged in the gastrointestinal tract–but that’s a known risk. In fact, there is very little unknown risk in these kinds of devices,” says Dr. Bettinger. “The breakfast you ate this morning is only in your GI tract for about 20 hours–all you need is a battery that can do its job for 20 hours and then, if anything happens, it can just degrade away.”
Bettinger and other researchers are exploring how minerals in a healthy diet, or even pigments from the skin or eye, could be used in bioelectronics. Ingestible devices that are used now are powered by off-the-shelf batteries, just like what you’d find in a watch. Bettinger challenges whether a segmented battery is necessary, as the natural liquids within the body can be the electrolytes that move current through the device. Labs have already proven that electronics built using this method can disintegrate in water after 2-3 months.
There’s also evidence that manufacturing biologically inspired “smart pills” can be cost-effective and pass regulatory approval. Ingestible medical devices and even 3D printed pills have been given the green light for patient use in recent years despite their atypical properties. Regarding cost, one of the reasons medications cost so much is that only a small percentage of a pill actually makes it to where it needs to be used in the body. Dr. Bettinger argues that if an electronic pill can make better use of expensive medications, then the amount needed for each patient can be reduced.
“There are many rapid advances in materials, inventions, and discoveries that can be brought to bear on medical problems,” Dr. Bettinger says. “If we can engineer devices that get the most mileage out of existing drugs, then that is a very attractive value proposition. I believe these devices can be tested in patients within the next 5-10 years.”
Illustration: Image is an artistic representation of edible electronics. –Christopher Bettinger.
Grant: Regenerative Medicine Therapy for Abdominal Aortic Aneurysm
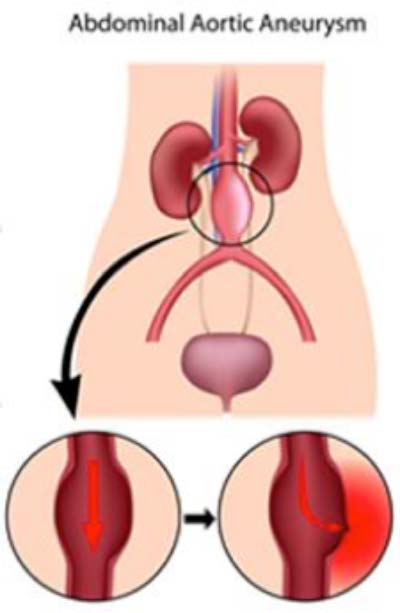 Abdominal aortic aneurysm (AAA), caused by the loss of elastin, a critical protein for blood vessel function, is responsible for approximately 10,000 American deaths every year. Through a grant from the National Institutes of Health (NIH), vascular bioengineering researchers at the University of Pittsburgh’s Swanson School of Engineering are proposing a new strategy for delivering therapeutic cells to the diseased cells in order to restore elastin levels and regenerate the aorta.
Abdominal aortic aneurysm (AAA), caused by the loss of elastin, a critical protein for blood vessel function, is responsible for approximately 10,000 American deaths every year. Through a grant from the National Institutes of Health (NIH), vascular bioengineering researchers at the University of Pittsburgh’s Swanson School of Engineering are proposing a new strategy for delivering therapeutic cells to the diseased cells in order to restore elastin levels and regenerate the aorta.
Funded through the NIH’s competitive Exploratory/Developmental Research Grant Award (R21) program, the research is being led by McGowan Institute for Regenerative Medicine faculty member David A. Vorp, PhD, Associate Dean for Research at the Swanson School and the William Kepler Whiteford Professor of Bioengineering. The proposal, “Outside-In Regenerative Therapy for Abdominal Aortic Aneurysm,” will receive $439,220 in direct and indirect funding through April 2017, and is a collaborative effort with John Curci, MD, a vascular surgeon at Vanderbilt University.
“Elastin is a highly elastic protein that allows soft tissues in our body – including blood vessels – to stretch and contract, but it is susceptible to the effects of aging, high blood pressure, high cholesterol, and smoking,” Dr. Vorp explained. “Therefore, abdominal aortic aneurysms greatly impact the elderly, especially men, and, if left untreated, can ultimately result in structural failure or rupture of the aortic wall and, many times, death.”
According to Dr. Vorp, the research will focus on development and delivery of mesenchymal stem cells to the outside of the aneurysm and will be tested in an established rodent model of the disease. Following treatment, the researchers will study whether the stem cells slow, halt, or even reverse the structural degeneration of the AAA. The results could eventually lead to an effective treatment for humans using a patient’s own stem cells.
“Few diseases present greater potential for regenerative cellular therapy than AAA, and the possibility of reconstitution and strengthening of the aorta is very exciting,” Dr. Vorp said. “By delivering stem cells to restore elastin, we can effectively treat a life-threatening disease without complex invasive surgery.”
Illustration: University of Pittsburgh Swanson School of Engineering.
Grant to Research Olfaction, Anosmia Awarded
 Researchers at the University of Pittsburgh are part of a multicenter team that has been awarded a $6.4 million, 3-year federal grant to figure out how the animal nose knows how to localize the smell of mates, food, and other significant scents. The co-principal investigators of the Pitt arm of the effort are McGowan Institute for Regenerative Medicine affiliated faculty member Bard Ermentrout, PhD, Distinguished University Professor of Computational Biology, a Professor of Mathematics, and an Adjunct Professor of Neurobiology at Pitt, and Nathan Urban, PhD, Professor in the Department of Neurobiology at Pitt School of Medicine and Associate Director of the University of Pittsburgh Brain Institute.
Researchers at the University of Pittsburgh are part of a multicenter team that has been awarded a $6.4 million, 3-year federal grant to figure out how the animal nose knows how to localize the smell of mates, food, and other significant scents. The co-principal investigators of the Pitt arm of the effort are McGowan Institute for Regenerative Medicine affiliated faculty member Bard Ermentrout, PhD, Distinguished University Professor of Computational Biology, a Professor of Mathematics, and an Adjunct Professor of Neurobiology at Pitt, and Nathan Urban, PhD, Professor in the Department of Neurobiology at Pitt School of Medicine and Associate Director of the University of Pittsburgh Brain Institute.
As part of its efforts under the federal BRAIN initiative, the National Science Foundation (NSF) is providing more than $15 million of funding for 17 scientists collaborating in 3 multi-institutional projects designed to explore the sense of smell, said Dr. Urban.
“We don’t really understand how the nose and brain enable a bloodhound to track a missing person, or rats to find landmines in Angola,” Dr. Urban said. “If we could understand how the olfactory system accomplishes this task, it could lead us to strategies to create artificial chemical detection systems. It also could be a model for understanding other sensory systems and the integration of multiple sensory cues.”
He added localizing where a smell is coming from is a very difficult problem to solve because it requires sampling odors at a distance from the source in turbulent air. So, the team includes experts in mathematics, the physics of airflow, neuroscience, and evolutionary biology to build models that quantify odors and develop algorithms of how they distribute in the environment, as well as to measure how animals and their brains react when exposed to odor plumes.
“We can localize sound in part because of differences between what the right and left ears hear,” explained Dr. Ermentrout. “Perhaps animals can orient by smell because of concentration differences picked up by each nostril, as well as incredibly rapid detection of increasing or decreasing intensities of odors. We intend to design mathematical models to examine these strategies.”
Dr. Ermentrout has reason to be curious about olfaction. Coincidentally, several months ago after a bad cold, the gourmand realized he couldn’t smell properly, which in turn has affected his ability to taste food. About one in five cases of anosmia develop after an upper respiratory tract infection.
“Anosmia can be a complication of neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease,” Dr. Urban noted. “In the future, we want to connect the dots and figure out why brain diseases can have these consequences.”
In addition to Drs. Urban and Ermentrout, the project’s other principal investigators are John Crimaldi, PhD, University of Colorado; Lucia Jacobs, PhD, University of California Berkeley; Jonathan Victor, MD, PhD, Weill Cornell Medical College; Katherine Nagel, PhD, New York University Medical Center; and Justus Verhagen, PhD, John Pierce Laboratory.
Illustration: Microsoft clipart.
Pitt Hosts Representatives of the DoD Regenerative Medicine Traveling Exchange Program
 McGowan Institute for Regenerative Medicine Associate Director Rocky Tuan, PhD, Director, Center for Cellular and Molecular Engineering, the Arthur J. Rooney, Sr. Professor and Executive Vice Chair, Department of Orthopaedic Surgery, and Co-Director of the Armed Forces Institute of Regenerative Medicine (AFIRM), the Department of Defense (DoD)-funded national, multi-institutional consortium, recently hosted a visit of the DoD Regenerative Medicine Traveling Exchange Program (TEP) to the University of Pittsburgh. This 1-day visit consisted of an overview of active AFIRM research projects being conducted at Pitt as well as presentations by Drs. Arthur Levine and Steven Shapiro representing Pitt’s School of Medicine and the University of Pittsburgh Medical Center (UPMC), respectively.
McGowan Institute for Regenerative Medicine Associate Director Rocky Tuan, PhD, Director, Center for Cellular and Molecular Engineering, the Arthur J. Rooney, Sr. Professor and Executive Vice Chair, Department of Orthopaedic Surgery, and Co-Director of the Armed Forces Institute of Regenerative Medicine (AFIRM), the Department of Defense (DoD)-funded national, multi-institutional consortium, recently hosted a visit of the DoD Regenerative Medicine Traveling Exchange Program (TEP) to the University of Pittsburgh. This 1-day visit consisted of an overview of active AFIRM research projects being conducted at Pitt as well as presentations by Drs. Arthur Levine and Steven Shapiro representing Pitt’s School of Medicine and the University of Pittsburgh Medical Center (UPMC), respectively.
The TEP aims to enhance the interaction between research-minded military physicians and researchers of leading academic health centers of the nation involved in AFIRM in areas that are of relevance to military medicine. In addition to Dr. Tuan, McGowan Institute for Regenerative Medicine AFIRM presenters included:
- William Wagner, PhD
- Stephen Badylak, DVM, PhD, MD
- Peter Rubin, MD
- Kacey Marra, PhD
- Yoram Vodovotz, PhD
- Vijay Gorantla, MD, PhD
TEP Fellows attending included:
- LCDR Matthew Bradley, Assistant Professor, Uniformed Services University of the Health Sciences
- Lt Col Megan Burgess, San Antonio Military Medical Center Center
- LCDR Daniel Grabo, Navy Trauma Training Center, LAC+USC Medical Center
- COL Booker King, Brooke Army Medical Center, San Antonio, Texas
- LTC John Oh, Chief, General Surgery Services, Walter Reed National Military Medical Center
Representing the DoD were: Dr. Wendy Dean, John Getz, and Lt Col Melinda Eaton.
Illustration: McGowan Institute for Regenerative Medicine faculty members Drs. Rocky Tuan (far left) and William Wagner (far right) and members of the DoD Regenerative Medicine Traveling Exchange Program.
Advancements in Wheelchair Technology Highlighted
 The University of Pittsburgh’s Human Engineering Research Laboratories Advanced Accessibility Engineering Project was named one of 20 advancements from the 2015 Innovation Festival, Washington, DC, 09/26-27/15, with potentially big implications for tomorrow. The Festival explored how today’s inventors are creating the world of our future.
The University of Pittsburgh’s Human Engineering Research Laboratories Advanced Accessibility Engineering Project was named one of 20 advancements from the 2015 Innovation Festival, Washington, DC, 09/26-27/15, with potentially big implications for tomorrow. The Festival explored how today’s inventors are creating the world of our future.
McGowan Institute for Regenerative Medicine affiliated faculty member Rory Cooper, PhD, showcased technology used to design, manufacture, and operate advanced wheelchairs.
He demonstrated advancements in wheelchair technology push rims, casters, backrests, and joysticks. Dr. Cooper is the FISA & Paralyzed Veterans of America (PVA) Chair and Distinguished Professor of the Department of Rehabilitation Science & Technology, and professor of Bioengineering, Physical Medicine and Rehabilitation, and Orthopedic Surgery at the University of Pittsburgh. Also, he is Founding Director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories, a VA Rehabilitation R&D Center of Excellence in partnership with Pitt. And, he is the Co-Director of the NSF Quality of Life Technology Engineering Research Center, a joint effort between Pitt and Carnegie Mellon University.
Festival participants were selected by experts in patented technology from the Intellectual Property Owners Organization, the American Intellectual Property Law Association, the National Academy of Inventors, and the independent inventor community.
MD Student Hopes to Dedicate a Year to Pursue a Career in Regenerative Medicine
 Adam McInnes, MD, was a 2014 participant in the inaugural McGowan Institute “Regenerative Medicine Summer School.” The Summer School week, endorsed by the Society for Biomaterials and the Tissue Engineering and Regenerative Medicine International Society (TERMIS), was initiated and continues to be led by McGowan Institute faculty member Bryan Brown, PhD, Research Assistant Professor with the Department of Bioengineering at the University of Pittsburgh with a secondary appointment in Pitt’s Department of Obstetrics, Gynecology and Reproductive Sciences.
Adam McInnes, MD, was a 2014 participant in the inaugural McGowan Institute “Regenerative Medicine Summer School.” The Summer School week, endorsed by the Society for Biomaterials and the Tissue Engineering and Regenerative Medicine International Society (TERMIS), was initiated and continues to be led by McGowan Institute faculty member Bryan Brown, PhD, Research Assistant Professor with the Department of Bioengineering at the University of Pittsburgh with a secondary appointment in Pitt’s Department of Obstetrics, Gynecology and Reproductive Sciences.
Regenerative medicine has been an interest of Dr. McInnes since he was 12 when saw the tissue engineering proof-of-concept Vacanti mouse on a TV show. Seeing how they grew this surgically-implanted ear using tissue engineering inspired him, and he knew there were so many more possibilities in this medical technology area. During the McGowan Institute Summer School week, using a combination of off-the-shelf biological scaffold materials and targeted physiotherapy, Dr. McInnes learned how researchers and clinicians were able to help trauma victims and veterans wounded in combat to regrow new muscle and tendon tissue and gain a much-improved quality of life. Dr. McInnes’ long-term career goal is to focus on regenerative medicine so he can help bring this exciting field to the bedside.
Dr. McInnes recently graduated from the University of Saskatchewan College of Medicine in Saskatoon, SK, Canada. Before beginning his residency training, he hopes to pursue a year-long effort to learn and do research in regenerative medicine. Currently, he has two opportunities from scientists at world-leading research institutes to study under their guidance. One is an offer at the Medical Research Council Centre for Regenerative Medicine in Edinburgh, UK, with Pierre Bagnaninchi, PhD, and Leonard Nelson, PhD. The second offer is at the McGowan Institute with deputy director Stephen Badylak, DVM, PhD, MD, Professor in the Department of Surgery and Director of the Center for Pre-Clinical Tissue Engineering within the McGowan Institute. For the purpose of these two research observerships, he will be considered a Visiting Scholar.
Dr. McInnes is very excited to take part in these learning opportunities and to explore his career in regenerative medicine. To do so, however, he needs funding help to make these prospects a reality. It is only after he meets his funding goal, will the research organizations extend to him a Visiting Scholar offer. Any monies raised will go towards his basic living expenses, travel expenses, conference fees, work visas, etc., while in the UK and the USA.
Because no known funding mechanism exists for his situation (he is no longer a student, an employed faculty member, researcher, nor licensed physician), Dr. McInnes has created an IndieGoGo page with details on how anyone can help him reach his immediate financial goal and his long-term regenerative medicine career goal. Additional details can be found on his Facebook page as well.
Best of luck, Dr. McInnes!
AWARDS AND RECOGNITION
Dr. Anna Balazs First Woman to Receive APS Polymer Physics Prize
 The 2016 American Physical Society (APS) Polymer Physics Prize will be awarded to McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical Engineering and the Robert v. d. Luft Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh. This annual prestigious accolade goes back to 1962 with Dr. Balazs being the very first woman to be awarded this prize since its inception over half of a century ago. The prize is given to “recognize outstanding accomplishment and excellence of contributions in polymer physics research.” Dr. Balazs’ prize consists of $10,000 and a certificate citing the contributions made by her. She will receive this recognition at the 2016 APS Annual Meeting, March 14-18, in Baltimore, Maryland.
The 2016 American Physical Society (APS) Polymer Physics Prize will be awarded to McGowan Institute for Regenerative Medicine affiliated faculty member Anna Balazs, PhD, Distinguished Professor of Chemical Engineering and the Robert v. d. Luft Professor, Department of Chemical & Petroleum Engineering, University of Pittsburgh. This annual prestigious accolade goes back to 1962 with Dr. Balazs being the very first woman to be awarded this prize since its inception over half of a century ago. The prize is given to “recognize outstanding accomplishment and excellence of contributions in polymer physics research.” Dr. Balazs’ prize consists of $10,000 and a certificate citing the contributions made by her. She will receive this recognition at the 2016 APS Annual Meeting, March 14-18, in Baltimore, Maryland.
“I have been inspired by the women polymer scientists before me and by my colleagues,” Dr. Balazs says. “I am humbled to receive this award.”
APS is a non-profit membership organization working to advance the knowledge of physics.
Congratulations, Dr. Balazs!
Dr. Anne Robertson Named One of 100 Inspiring Women in STEM by INSIGHT Into Diversity Magazine
 McGowan Institute for Regenerative Medicine affiliated faculty member Anne M. Robertson, PhD, Professor of Mechanical Engineering and William Kepler Whiteford Professor at the University of Pittsburgh’s Swanson School of Engineering, was named a recipient of the 100 Women in STEM Award by INSIGHT Into Diversity magazine.
McGowan Institute for Regenerative Medicine affiliated faculty member Anne M. Robertson, PhD, Professor of Mechanical Engineering and William Kepler Whiteford Professor at the University of Pittsburgh’s Swanson School of Engineering, was named a recipient of the 100 Women in STEM Award by INSIGHT Into Diversity magazine.
According to the magazine, the award is being presented “as a tribute to 100 women whose work and achievements not only encourage others in their individual STEM fields, but also inspire a new generation of young women to consider careers in science, technology, engineering, and math. These remarkable women continue to make a significant difference through mentoring and teaching, research, and other efforts worthy of this national recognition.” Dr. Robertson was nominated by Ann E. Thompson, MD, Vice Dean of the University of Pittsburgh School of Medicine, and Professor of Critical Care Medicine and Pediatrics.
Dr. Robertson was the first woman hired into a tenure-track position in the Swanson School’s Department of Mechanical Engineering and served as Director of the Graduate Program in Mechanical Engineering from 2004-2008. She is currently Director of the newly formed Center for Faculty Excellence in the Swanson School.
Dr. Robertson leads a research team that investigates cerebral aneurysms, which are pathological outcroppings of brain arteries that can lead to fatal brain hemorrhages. She is the recipient of two coveted National Institutes of Health R21 grants to study the link between hemodynamics and wall structure in cerebral aneurysms. The team’s long-term objectives are to establish new pharmacological-based treatment methods for cerebral aneurysms and improve clinical treatments that function by altering flow in the aneurysm dome. She is also co-PI on a third R21 developing tissue engineering blood vessels, in collaboration with the Swanson School’s Yadong Wang, PhD (a McGowan Institute for Regenerative Medicine faculty member), and Paolo Zunino, PhD.
In 2007, Dr. Robertson was awarded the Beitle-Veltri Memorial Outstanding Teaching Award, given annually to one faculty member in the Swanson School of Engineering at the University of Pittsburgh as well as the Robert O. Agbede Faculty Award for Diversity, in recognition of significant contributions to enhancing and supporting diversity in the School of Engineering. She is also a member of the faculty in the Department of Bioengineering. She earned her BS in mechanical engineering from Cornell University and her MS and PhD in mechanical engineering from the University of California at Berkeley, where she was also a President’s Postdoctoral Fellow in the Department of Chemical Engineering.
ASME Honors Dr. Savio L-Y. Woo With Medal Recognition
 A University of Pittsburgh professor who himself has received numerous national and international accolades – among them an Olympic gold medal – will now have an award medal struck in his honor.
A University of Pittsburgh professor who himself has received numerous national and international accolades – among them an Olympic gold medal – will now have an award medal struck in his honor.
Established by the American Society of Mechanical Engineers (ASME) on the recommendation of its Bioengineering Division, the award celebrates the career and achievements of bioengineering trailblazer Savio L-Y. Woo, PhD, a Distinguished University Professor of Bioengineering in the University of Pittsburgh’s Swanson School of Engineering, the founder and director of the Musculoskeletal Research Center (MSRC) at Pitt, and a faculty member of the McGowan Institute for Regenerative Medicine. The ASME Savio L-Y. Woo Translational Biomechanics Medal will be a Society-level award to recognize ASME members who have translated meritorious bioengineering science to clinical practice through research, education, professional development, and service to the bioengineering community.
“The medal is really a recognition of the success of many: my prized pupils that I have taught (and they have taught me!); my junior colleagues that I have mentored; my good and kind friends and co-workers that I am fortunate to know and to learn from; and most importantly, my supportive and loving family: Pattie, Kirstin, Jonathan, Adam, Zadie, and Arden,” Dr. Woo said.
Prior to the creation of the Woo Medal, ASME had three awards honoring contributions to the field of bioengineering: the Y.C. Fung Young Investigator Award, the Van C. Mow Medal for mid-career researchers, and the H.R. Lissner Medal for career achievement. Unlike those awards, which focus on research contributions to bioengineering and engineering, the new award is intended to recognize the significant contributions of bioengineers whose work has resulted in the development of a medical device or equipment, contributed to new approaches of disease treatment, or established new injury treatment modalities.
“Dr. Woo has been a leader in improving orthopedic surgery and patient outcomes through scientific research and engineering design,” said Sara E. Wilson, PhD, director of the Bioengineering Graduate Program and associate professor of mechanical engineering at the University of Kansas and chair of the ASME Bioengineering Division. “This award recognizes the unique contributions of those that bridge the gap between research and clinical practice.”
“It was very special for me to take part in establishing this legacy in honor of Dr. Savio L-Y. Woo,” added Jennifer S. Wayne, PhD, the former chair of the Bioengineering Division who spearheaded the effort to establish the award. “It forever acknowledges what he has accomplished for the bioengineering field and the ASME Bioengineering Division.” The award received the full support of the Bioengineering Division’s leadership, with David A. Vorp, PhD, associate dean for research, the William Kepler Whiteford Professor of Bioengineering at Pitt’s Swanson School of Engineering, and McGowan Institute for Regenerative Medicine faculty member; and Matthew J. Gounis, PhD, associate professor of radiology at the University of Massachusetts Medical School, both recent chairs of the Bioengineering Division, having crucial parts in the approval process.
Candidates for the new award must be active members of the Bioengineering Division. The award consists of $1,000, a bronze medal, a certificate, and a travel expense supplement to attend the award presentation. Nominations for the first Woo Medal are being accepted through October 1. The Bioengineering Division expects to present the first award next summer, during the Summer Biomechanics, Bioengineering and Biotransport (SB3C) Conference in National Harbor, Maryland.
Dr. Kathryn Whitehead: Searching for a Nanoparticle in a Haystack
 Every year, Popular Science honors the 10 brightest young minds that are reshaping science, engineering, and the world. McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, assistant professor of chemical engineering and biomedical engineering at Carnegie Mellon University, was named one of the magazine’s 2015 honorees. She is a member of “The Brilliant 10” for her innovative work on drug delivery systems, for designing nanoparticles that treat disease by delivering therapeutic drugs to specific areas in the body. Her research will revolutionize how we treat formidable diseases, such as cancer, diabetes, and hereditary disorders.
Every year, Popular Science honors the 10 brightest young minds that are reshaping science, engineering, and the world. McGowan Institute for Regenerative Medicine affiliated faculty member Kathryn Whitehead, PhD, assistant professor of chemical engineering and biomedical engineering at Carnegie Mellon University, was named one of the magazine’s 2015 honorees. She is a member of “The Brilliant 10” for her innovative work on drug delivery systems, for designing nanoparticles that treat disease by delivering therapeutic drugs to specific areas in the body. Her research will revolutionize how we treat formidable diseases, such as cancer, diabetes, and hereditary disorders.
During her career, Dr. Whitehead has synthesized and tested nearly 5,000 nanoparticle delivery vehicles en route to identifying a select few that potently shuttle drugs into exactly the right cells. This feat was challenging, in part, because the body’s immune system considers therapeutic nanoparticles to be foreign substances that need to be destroyed. However, Dr. Whitehead’s nanoparticles circumvent the immune system and are free to deliver medicine to cells in many parts of the body, including the liver, the skin, and the intestine. Dr. Whitehead’s research group is now using her nanoparticles to engineer therapies for maladies that include inflammatory bowel disease, chronic wounds, and Non-Hodgkin’s lymphoma, a type of blood cancer.
“Cancer therapy is so difficult for patients, in large part, because of the toxic side effects of chemotherapy,” Dr. Whitehead said. “In contrast, our targeted nanoparticles deliver drugs only to cancerous tissue, sparing healthy cells. We expect these targeted treatments to extend the lives of cancer patients while increasing their quality of life through a reduction in side effects.”
Dr. Whitehead’s approach to finding the right nanoparticles for drug delivery was unorthodox in that it required her to examine a very large number of nanoparticles using high-throughput screening.
“Although high-throughput screening has not been a well-accepted approach to scientific discovery, I felt strongly that we needed to test many compounds to maximize our chances of success,” Dr. Whitehead said. Her hard work has paid off in the discovery of these versatile nanoparticles, and she has broadened the scientific community’s understanding of how drug delivery chemistry affects efficacy. She is now able to predict which nanoparticles will work in living animals.
Dr. Whitehead credits much of her scientific success to plain old perseverance; her lab’s mascot is the honey badger, an animal known for its determination. “People look at me like I’m crazy when I say I tested thousands of materials,” she says. “I think some others would have given up.”
Congratulations, Dr. Whitehead!
Dr. Steven Little Recognized
 At the University of Pittsburgh, McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, has been appointed the William Kepler Whiteford Endowed Professor for a term of 5 years. This appointment is in recognition of Dr. Little’s many outstanding achievements in research, teaching, and service, as well as of his exceptional leadership as Chair, Department of Chemical and Petroleum Engineering.
At the University of Pittsburgh, McGowan Institute for Regenerative Medicine faculty member Steven Little, PhD, has been appointed the William Kepler Whiteford Endowed Professor for a term of 5 years. This appointment is in recognition of Dr. Little’s many outstanding achievements in research, teaching, and service, as well as of his exceptional leadership as Chair, Department of Chemical and Petroleum Engineering.
Researchers in Dr. Little’s Lab focus upon therapies that are biomimetic in that they replicate the biological function and interactions of living entities using synthetic systems. The areas of study include bioengineering, chemistry, chemical engineering, ophthalmology, and immunology. The health issues addressed include autoimmune disease, battlefield wounds, cancer, HIV, ocular diseases, and transplantation.
To date this calendar year, and in addition to the William Kepler Whiteford Endowed Professorship, Dr. Little has been appointed as a University Honors College – Faculty Fellow; elected, Fellow, Biomedical Engineering Society; received the Curtis W. McGraw Research Award from ASEE; received The Carnegie Science Award (Advanced Materials); and was selected one of the Pittsburgh Business Times’ Fast Trackers (University Leaders).
Congratulations, Dr. Little!
Regenerative Medicine Podcast Update
 The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#152 –– Dr. Bryan Brown is the Director of the McGowan Institute Annual Summer School and an Assistant Professor in the Department of Bioengineering at the University of Pittsburgh. Dr. Adam McInnes is an alumnus of the Summer School. Drs. Brown and McInnes discuss the Summer School and their future plans in pursuing regenerative medicine.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PICTURE OF THE MONTH
The Picture of the Month is a compliment to the longstanding features Grant of the Month and Publication of the Month. Each of these features highlights the achievements of McGowan affiliated faculty and their trainees. As we have always welcomed suggestions for grants and publications, please also consider submitting images that can highlight your pioneering work.
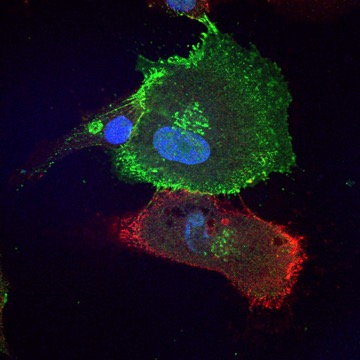
Cadherin expression distinguishes corneal stem cells from their more differentiated and less stem-like daughter cells. The more primitive red cells expresses N-cadherin, and the green cell expresses cadherin 11, with a less stem-like phenotype.
Image from James Funderburgh, Fox Center for Vision Restoration
PUBLICATION OF THE MONTH
Author: Shaffiey SA, Jia H, Keane T, Costello C, Wasserman D, Quidgley M, Dziki J, Badylak S, Sodhi CP, March JC, Hackam DJ.
Title: Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals.
Summary: AIMS: To investigate the growth and differentiation of intestinal stem cells on a novel tubular scaffold in vitro and in vivo.
METHODS: Intestinal progenitor cells from mice or humans were cultured with myofibroblasts, macrophages and/or bacteria, and evaluated in mice via omental implantation. Mucosal regeneration was evaluated in dogs after rectal mucosectomy followed by scaffold implantation.
RESULTS: Intestinal progenitor cells differentiated into crypt-villi structures on the scaffold. Differentiation and scaffold coverage was enhanced by coculture with myofibroblasts, macrophages and probiotic bacteria, while the implanted scaffolds enhanced mucosal regeneration in the dog rectum.
CONCLUSION: Intestinal stem cell growth and differentiation on a novel tubular scaffold is enhanced through addition of cellular and microbial components, as validated in mice and dogs.
Source: Regen Med. 2015 Sep 23. [Epub ahead of print]
GRANT OF THE MONTH
PI: David Vorp
Co-PI: John Curci
Title: Outside-In Regenerative Therapy for Abdominal Aortic Aneurysm
Description: Few diseases represent the optimal potential target for regenerative cellular therapy more than the abdominal aortic aneurysm (AAA). A disease that affects a large number of elderly in the United States with a natural history that results in structural failure of the aortic wall and death, AAA continues to represent a critical need for biologic therapy. Regenerative therapy consisting of the delivery of stem cells to the damaged aorta presents a conceptually strong opportunity for the reconstitution of the aortic mural matrix and therefore aortic strength – any test of such a therapy must be done on an established aneurysm to most accurately represent what occurs in the clinic. In this proposal, we have combined the strengths of two laboratories with complementary scientific capability, and with a common interest in the development of effective biologic therapies for AAA disease. The early product of this collaborative pairing is published “proof of concept” evidence that mesenchymal stem cell (MSC) delivery to the wall of a murine AAA can slow progression. The purpose of this R21 Exploratory/Developmental Research Proposal is to develop a clinically- translatable MSC delivery system that would result in aortic matrix repair and regeneration. Our hypothesis is that local stem cell delivery to a murine AAA via an adventitially-applied hydrogel and magnetic assistance will result in intramural cell engraftment, matrix repair, and mechanical stabilization of the aortic wall. To address our hypothesis, we will execute the following specific aims: Specific Aim 1 is to develop and validate a technique to deliver MSCs into the aortic wall periadventitially using a hydrogel vehicle and magnetic guidance. The technique will be optimized by testing a cadre of iron nanoparticle types, fibrin hydrogel formulations, and stem cell concentrations both in vitro and in vitro. Specific Aim 2 is to demonstrate that local MSC delivery halts and reverses the functional and structural degeneration of an AAA in an established rodent model. MSC hydrogels developed in Specific Aim 1 will be applied to the adventitia of an elastase-induced model AAA after allowing for varying degrees of matrix degeneration. Metrics for success of the various therapies versus cell-free hydrogel controls on aortic tissue will involve: i) functional assessment (including aortic diameter and biomechanical parameters) and ii) detailed microstructural and cellular composition assessment. The expected outcome of this work is the development and proof-of-concept of a new technology for stem cell delivery to AAA.
Source: National Heart, Lung, and Blood Institute
Term: July 15, 2015 – April 30, 2017
Amount: $206,747
