McGowan Institute for Regenerative Medicine affiliated faculty member Shawn Bengtson, RQAP-GLP, Director, Quality Assurance-Quality Systems Programs, with the assistance of Nika Hazen, Quality Systems Development Manager, played a key role in the University of Pittsburgh Center for Vaccine Research’s (CVR’s) pursuit of improved COVID-19 medical countermeasures and therapeutic technologies.
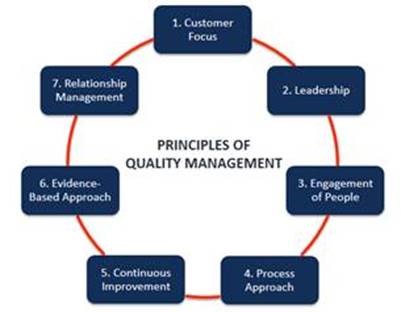
The McGowan Institute Center for Preclinical Studies (CPCS) under Mr. Bengtson’s leadership developed a quality management program initially to address needs in the CPCS. However, the unique procedures and training that a formal quality management program requires has led to requests from other University of Pittsburgh departments for assistance in the development and implementation of their own Quality Management Systems.
The CVR Regional Biocontainment Laboratory (RBL) had a need to initiate the establishment of a quality management program as their work on COVID-19 and associated variant testing ramped-up in 2020. Mr. Bengston and Ms. Hazen were instrumental in the design and implementation of the CVR-RBL quality management program addressing the challenges of a select agent Biosafety Level 3 regulated research environment (Biosafety Level 3 and Animal Biosafety Level 3 laboratories).
In addition to the CVR-RBL support, the McGowan Institute Quality Assurance Unit is assisting other programs across the campus, such as the Infectious Disease Laboratory, by providing systems implementation and monitoring support along with serving as the Quality Assurance Advisory Core for the NIH/NIDCR funded Michigan-Pitt-Wyss Interdisciplinary Translational Project Program and the SHARE/MediCiti Institute of Medical Sciences, India.
Illustration: Shawn Bengtson