Biomarkers For The Diagnosis Of ALS
Inventors: Robert Bowser Ph.D.
Patent Number: 12/899,235
Abstract
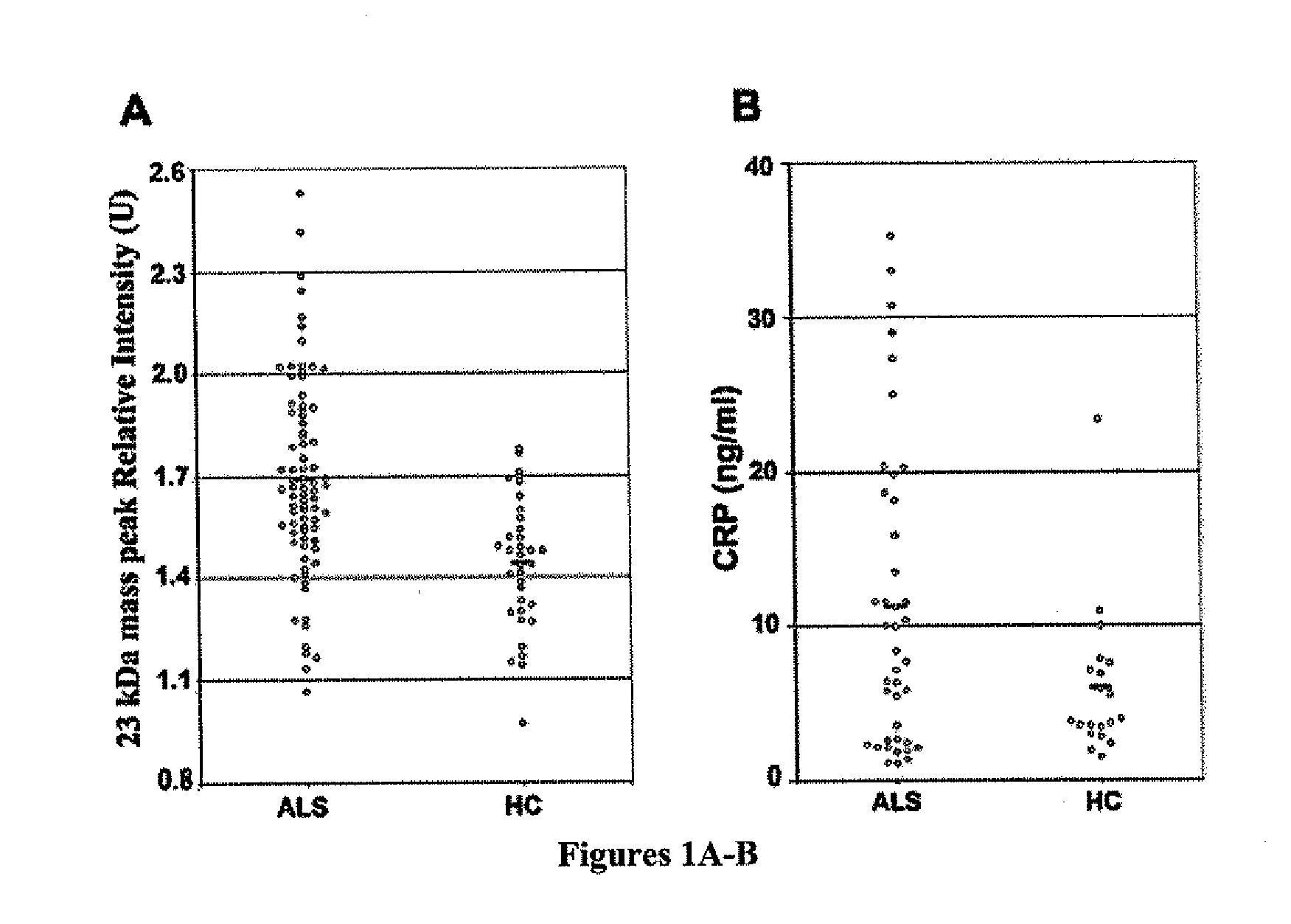
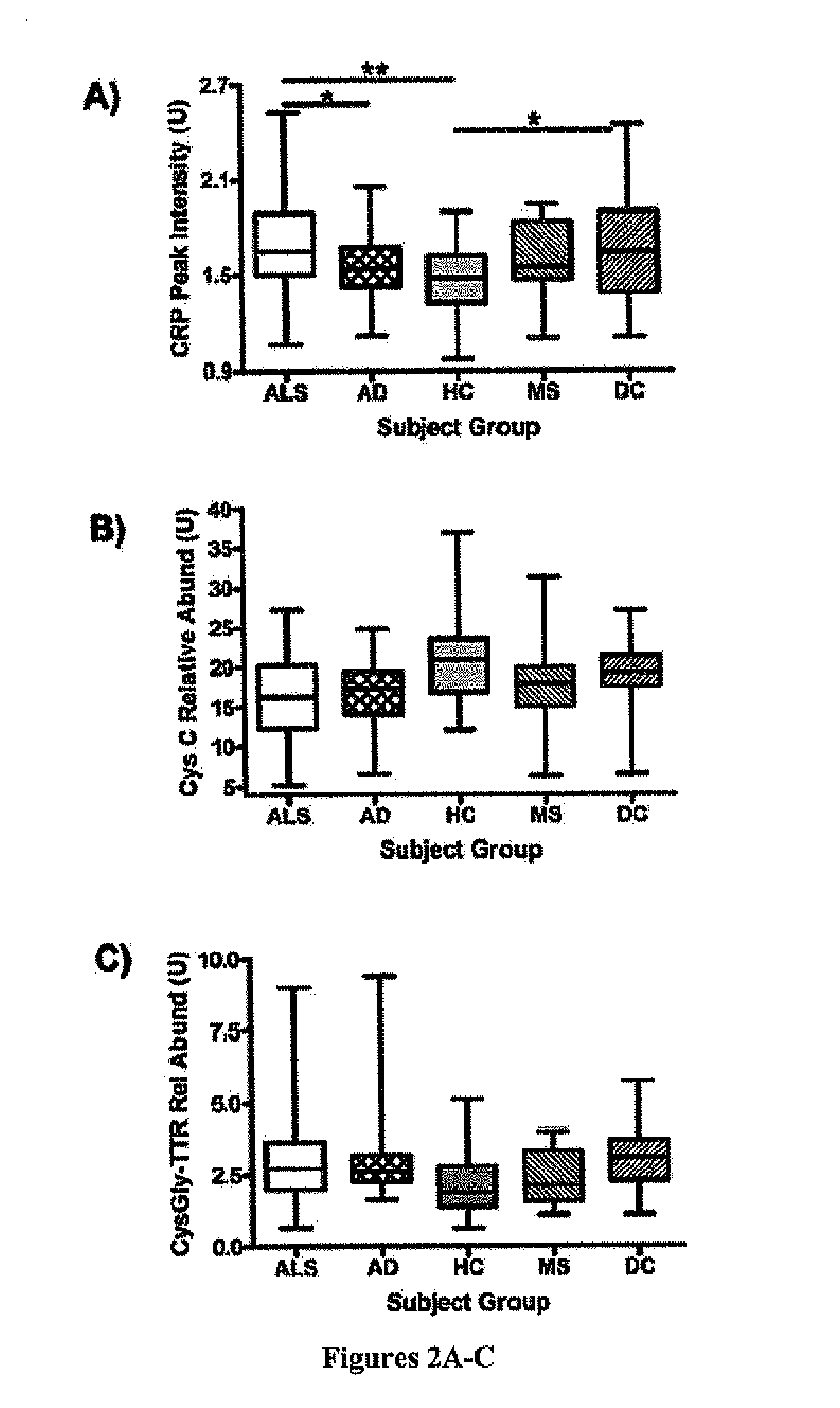
Methods for determining the onset of ALS in a subject are provided. One method includes analyzing a sample obtained from the subject for the presence or amount of one or more biomarkers indicative of ALS. In a preferred embodiment, the biomarkers are one or more of the following: C-reactive protein (CRP), cystatin c, plasminogen, complement C3, CysGly-transthyretin, and phosphorylated neurofilament heavy chain (pNFH). The sample is typically cerebral spinal fluid (CSF). The levels or concentrations of the biomarkers can be used to determine the onset of ALS, monitor the progression of ALS, or monitor the progression of a treatment for ALS.