December 2020 | VOL. 19, NO. 12| www.McGowan.pitt.edu
Synthetic Biology and Machine Learning Speed the Creation of Lab-Grown Livers

Researchers at the University of Pittsburgh School of Medicine have combined synthetic biology with a machine-learning algorithm to create human liver organoids with blood- and bile-handling systems. When implanted into mice with failing livers, the lab-grown replacement livers extended life.
The study, published in Cell Systems, shows that it’s possible to trigger and speed up the maturation of a lab-grown organ without sacrificing precision or control.
“Pregnancy is nine months—it takes that long and even months after birth for new organs to mature—but if a person needs a liver, they may not be able to wait that long,” said study author Mo Ebrahimkhani, MD, associate professor of pathology and bioengineering, and member of the Pittsburgh Liver Research Center and the McGowan Institute for Regenerative Medicine. “We showed it’s possible to get human liver tissue with four main cell types and vasculature in 17 days. We can mature tissue almost to the third trimester in only three months.”
Other groups have attempted to coax organoid maturation in a dish using growth factors, but it’s expensive, inconsistent and prone to human error, Dr. Ebrahimkhani said. Often, there are unwanted tissue or cell types—such as intestine or brain cells growing in the middle of what should be solid liver.
Using genetic engineering is cleaner but also more complex to orchestrate. So, Dr. Ebrahimkhani partnered with Patrick Cahan, PhD, at Johns Hopkins University to use a machine-learning system that can reverse engineer the genes necessary for human liver maturation.
Then, Dr. Ebrahimkhani together with his collaborator at Pitt, Samira Kiani, MD, applied genetic engineering techniques, including CRISPR, to turn a mass of immature liver tissue—originally derived from human stem cells—into what the team calls “designer liver organoids.” Dr. Kiani is a visiting associate professor, Liver Research Center, Department of Pathology, School of Medicine, University of Pittsburgh, and an affiliated faculty member of the McGowan Institute.
The more mature the organoids got, the more capillaries and rudimentary bile duct cells snaked their way through the thin sheet of tissue, and the more closely the function of the tiny organ rivaled its full-size natural human model. Energy storage, fat accumulation, chemical transport, enzyme activity and protein production were all closer to adult human liver function, though still not a perfect match.
Dr. Ebrahimkhani imagines designer organoids having three main uses: drug discovery, disease modeling, and organ transplant. Since the stem cells can come from the patient’s own body, lab-grown organs could be personalized, so there would be no threat of immune rejection.
When transplanted into mice with damaged livers, Dr. Ebrahimkhani’s designer liver organoids successfully integrated into the animals’ bodies and continued to work—producing human proteins that showed up in the animals’ blood and prolonging the animals’ lives.
This is a proof-of-principle to show that it’s possible, Dr. Ebrahimkhani said. The technique could potentially go much further.
“Our reference was a nature-designed human liver, but you can go after any design you like. For instance, you can make a genetic switch that protects the tissue from a virus, target the DNA of the virus and destroy it,” Dr. Ebrahimkhani said. “That sets this method apart.”
Additional authors on the study include co-first authors Jeremy Velazquez and Ryan LeGraw, as well as Farzaneh Moghadam, PhD, Joseph Maggiore, Joshua Hislop and Silvia Liu, PhD, of Pitt; Yuqi Tan of Johns Hopkins; Jacquelyn Kilbourne and Christopher Plaisier, PhD, of Arizona State University; and Davy Cats and Susana Chuva de Sousa Lopes, PhD, of Leiden University Medical Center.
Several of the authors have submitted a patent (WO2019237124) for the methods described in this paper.
RESOURCES AT THE MCGOWAN INSTITUTE
January Histology Special
Safranin O staining is used for the detection of cartilage, mucin, and mast cell granules on formalin-fixed, paraffin-embedded tissue sections or frozen sections. The cartilage and mucin will be stained orange to red, and the nuclei will be stained black. The background is stained bluish green.
You’ll receive 30% off Safranin O staining in January when you mention this ad. Contact Julia at the McGowan Core Histology Lab by email: Hartj5@upmc.edu or call 412-624-5265.
New Sample Submission Procedures: In response to COVID-19, we ask that you contact us to schedule a drop off time. When you arrive at the building you can call our laboratory at (412)624-5365. Someone will meet you in the lobby to collect your samples. When your samples are completed, you will receive an email to schedule a pickup time.
SAVE THE DATE
 McGowan Institute Annual Retreat
McGowan Institute Annual Retreat
Save the dates of March 9, 10 and 11, 2021 for the McGowan Institute Scientific Retreat. The program committee, under the leadership of Bryan Brown, PhD, is developing an exciting program. The format will be virtual.
Updates will follow soon.
SCIENTIFIC ADVANCES
Dr. Nathan Bahary Expands on Recent Advances with Actionable Alterations Spanning GI Cancers

McGowan Institute for Regenerative Medicine affiliated faculty member Nathan Bahary, MD, PhD, associate professor in the Department of Medicine, Division of Oncology at the University of Pittsburgh, and a medical oncologist and hematologist at the University of Pittsburgh Medical Center Hillman Cancer Center, discusses with Erica DiNapoli for OncLive the importance of seeking targetable alterations in gastrointestinal (GI) cancers, as well as recent advancements in the space.
The emergence of actionable alterations has allowed the field of GI cancer to shift away from traditional chemotherapy approaches to the adoption of more personalized treatment approaches, according to Dr. Bahary, who added that these targeted therapies are now being explored in combination with different immunotherapies to elicit stronger, more durable responses.
“Targeted therapies have helped different subsets of patients, whether that is a larger subset of patients with intrahepatic cholangiocarcinoma or a smaller subset of those with pancreatic cancer. However, in mismatch repair—deficient colorectal cancer—several patients still do not respond,” Dr. Bahary explained. “In an attempt to help them achieve better and longer-lasting responses, ongoing research efforts are now focused on integrating the use of immunotherapies with targeted therapies. This approach has previously shown great promise in hepatocellular carcinoma and other GI tumors.”
The interview with OncLive was held during the 2020 Institutional Perspectives in Cancer webinar on Precision Medicine. Read a transcript of that conversation here.
Overlooked Treatment May Improve Survival Rates for Ovarian Cancer Patients

A treatment that may improve the 10-year survival rate for ovarian cancer patients should be a consideration, according to a recent study led by McGowan Institute for Regenerative Medicine affiliated faculty member Faina Linkov, PhD, MPH, chair of Duquesne University’s Health Administration and Public Health program in the Rangos School of Health Sciences.
Intraperitoneal/intravenous chemotherapy (IP/IV) has been associated with improved survival rates in several published randomized trials. Its use in the U.S. and Europe, however, has been limited by various complications, including harsh side effects reported by some patients, catheter complications and lower rates of completion. Additionally, IP/IV chemotherapy is not always available and accessible outside of larger tertiary healthcare facilities.
The recent Duquesne/University of Pittsburgh study, published in Cancer Medicine, reported that those who underwent the IP/IV treatment experienced improved 10-year survival rates in comparison to IP/IV treatment-eligible patients who did not undergo the treatment. Typically, less than 50 percent of patients survive five years after being diagnosed with advanced ovarian cancer. This study is unique in evaluating long-term ovarian cancer survivors.
“The study shows that IP/IV chemotherapy should be considered for ovarian cancer patients who are good candidates for the procedure,” said Dr. Linkov. “While the study found that only 14 percent of patients were treated with IP/IV, they did experience improved 10-year survival.”
Using hospital registry data from the UPMC Health system, Dr. Linkov analyzed the data of more than 1,800 ovarian cancer patients and worked with gynecologic oncologists to interpret the results. The IP/IV treatment was significantly associated with improved 10-year survival rates, with no impact on cancer recurrence.
“The study suggests that we need to explore new ways of identifying patients who may benefit from this treatment,” said Dr. Linkov.
3D Bioprinted Heart Provides New Tool for Surgeons
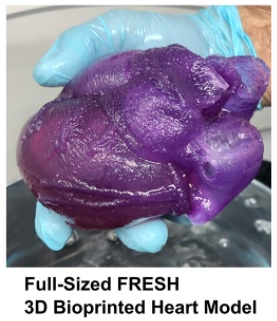
The first full-size 3D bioprinted human heart model using the Freeform Reversible Embedding of Suspended Hydrogels (FRESH) technique has been created by Carnegie Mellon University’s (CMU’s) Adam Feinberg, PhD, and his team. The model, created from MRI data using a specially built 3D printer, realistically mimics the elasticity of cardiac tissue and sutures. This milestone represents the culmination of two years of research, holding both immediate promise for surgeons and clinicians, as well as long-term implications for the future of bioengineered organ research.
The FRESH technique of 3D bioprinting was invented in Dr. Feinberg’s lab to fill an unfilled demand for 3D printed soft polymers, which lack the rigidity to stand unsupported as in a normal print. FRESH 3D printing uses a needle to inject bioink into a bath of soft hydrogel, which supports the object as it prints. Once finished, a simple application of heat causes the hydrogel to melt away, leaving only the 3D bioprinted object.
While Dr. Feinberg, a professor of biomedical engineering and materials science and engineering at CMU and an affiliated faculty member of the McGowan Institute for Regenerative Medicine, has proven both the versatility and the fidelity of the FRESH technique, the major obstacle to achieving this milestone was printing a human heart at full scale. This necessitated the building of a new 3D printer custom made to hold a gel support bath large enough to print at the desired size, as well as minor software changes to maintain the speed and fidelity of the print.
Major hospitals often have facilities for 3D printing models of a patient’s body to help surgeons educate patients and plan for the actual procedure; however, these tissues and organs can only be modeled in hard plastic or rubber. Dr. Feinberg’s team’s heart is made from a soft, natural polymer called alginate, giving it properties similar to real cardiac tissue. For surgeons, this enables the creation of models that can cut, suture, and be manipulated in ways similar to a real heart. Dr. Feinberg’s immediate goal is to begin working with surgeons and clinicians to fine tune their technique and ensure it’s ready for the hospital setting.
“We can now build a model that not only allows for visual planning, but allows for physical practice,” says Dr. Feinberg. “The surgeon can manipulate it and have it actually respond like real tissue, so that when they get into the operating site they’ve got an additional layer of realistic practice in that setting.”
The paper, published in ACS Biomaterials Science and Engineering, represents another important marker on the long path to bioengineering a functional human organ. Soft, biocompatible scaffolds like that created by Dr. Feinberg’s group may one day provide the structure onto which cells adhere and form an organ system, placing biomedicine one step closer to the ability to repair or replace full human organs.
“While major hurdles still exist in bioprinting a full-sized functional human heart, we are proud to help establish its foundational groundwork using the FRESH platform while showing immediate applications for realistic surgical simulation,” added Eman Mirdamadi, lead author on the publication and former student of Dr. Feinberg.
The research paper was also co-authored by Dr. Feinberg’s students Joshua W. Tashman, Daniel J. Shiwarski, PhD, and Rachelle N. Palchesko, PhD.
Illustration: The Feinberg Lab. Carnegie Mellon University.
Oncorus Has ‘Largest IPO’ Out of Any Pitt Spinout

Oncorus, a viral immunotherapies company focused on driving innovation to transform outcomes for cancer patients and a company spun out of the University of Pittsburgh, recently initiated an initial public stock offering, raising nearly $90 million as it enters clinical trials on its oncolytic virus cancer therapy. That amount is being called the “largest IPO of a Pitt spinout” by the University’s Innovation Institute.
The technology for the Cambridge, Massachusetts-based company was licensed from Pitt from the lab of Joseph Glorioso, PhD, professor of microbiology and molecular genetics in Pitt’s School of Medicine and an affiliated faculty member of the McGowan Institute for Regenerative Medicine. Dr. Glorioso was one of the founders of Oncorus and is chair of the company’s scientific advisory board. The company, founded in 2016, had previously raised approximately $140 million in private investment.
“It’s great. It’s a substantial amount of money,” Dr. Glorioso said. “It’s really based on not only the technology within the company being tested in patients, but also other technologies that are very exciting and will be used to treat people with cancer.”
COVID-19 Response Update with Dr. Derek Angus
In the midst of rising COVID-19 case rates globally, Derek Angus, MD, MPH, and Rochelle Walensky, MD, MPH, return to JAMA’s Q&A series to update viewers on developments in the pandemic and the critical care management of COVID-19 patients. Dr. Angus is Chief Health Care Innovation Officer at the University of Pittsburgh Medical Center, professor and chair of Critical Care Medicine, and a senior JAMA editor. Dr Walensky is Chief of Infectious Diseases at Massachusetts General Hospital and a professor at Harvard Medical School. This program was recorded November 19, 2020.
Drs. Angus and Rochelle spoke with Howard Bauchner, MD, JAMA Editor-in-Chief, about:
- Current standard of care for critically ill patients with respiratory failure
- Current therapies, i.e., steroids, Remdesivir, Toci, monoclonal antibodies, cyclosporine, interferon beta
- Current extraordinary number of COVID cases, strained hospital systems/resources including the workforce
- Potential vaccines from Pfizer/BioNTech and Moderna
Watch a video of this discussion here.
Listen to an audio recording of this discussion here.
Illustration: UPMC.
Positive Effects of Physical Activity in Bariatric Surgery Patients

In a recent issue of the Annals of Surgery, epidemiologists from the University of Pittsburgh published an analysis that could help guide clinicians and policymakers in counseling bariatric surgery patients to improve their quality of life for many years to come. McGowan Institute for Regenerative Medicine affiliated faculty member Steven Belle, PhD, MScHyg, professor in the Department of Epidemiology and a co-director in the Epidemiology Data Center in the Graduate School of Public Health at the University of Pittsburgh, is a co-author on the study.
First author Wendy King, PhD, associate professor of epidemiology at Pitt’s Graduate School of Public Health, found that higher physical activity levels after bariatric surgery lessen depressive symptoms and improve mental and physical quality of life, irrespective of weight loss.
Every year, tens of thousands of Americans who struggle with obesity undergo gastric bypass surgery to manage their body weight and comorbidities, such as diabetes. Yet, the Pitt scientists found, while most patients are at least somewhat satisfied with their surgery long-term, satisfaction decreased from 85% to 77% three to seven years post-surgery. Most patients also continue to lead sedentary lives, which contributes to weight regain and negatively affects their mental well-being.
“Our data support why it’s important to counsel patients regarding their physical activity behaviors,” said Dr. King. “Although patients in general are not meeting physical activity recommendations post-surgery, we found a dose-response relationship – the more active patients were, the better improvement they had in depressive symptoms and health-related quality of life. Every bit matters.”
Dr. King found that improvements in mental and physical health-related quality of life differed by physical activity level. By analyzing objective measures collected from wearable activity monitors — step count, amount of time spent sedentary and amount of time spent doing moderate-to-vigorous activity — she found that higher levels of physical activity related to improvements independent of weight loss. Dr. King showed that higher activity level predicted better weight loss and less weight regain – but that study didn’t look at quality-of-life measures.
Even after the surgery, an average bariatric surgery patient leads a significantly more sedentary lifestyle than recommended by physicians.
Dr. King says this may explain why the magnitude of associations between physical activity level and improvement in health-related quality of life and depressive symptoms in their cohort was small. Still, the findings provide support for expanding measures that increase physical activity in bariatric surgery patients to influence mental and physical health outcomes.
“Most insurance providers include coverage for dietary counseling but don’t reimburse expenses for hiring a health coach or getting a gym membership,” said Dr. King. “There needs to be more systemic support to help patients increase their activity level and maintain an active lifestyle post-surgery.”
The study analyzed data collected from 1,700 adults who underwent Roux-en-Y gastric bypass surgery between March 2006 and April 2009 and were followed for up to seven years.
AWARDS AND RECOGNITION
Dr. Rory Cooper Appointed Assistant Vice Chancellor for Research
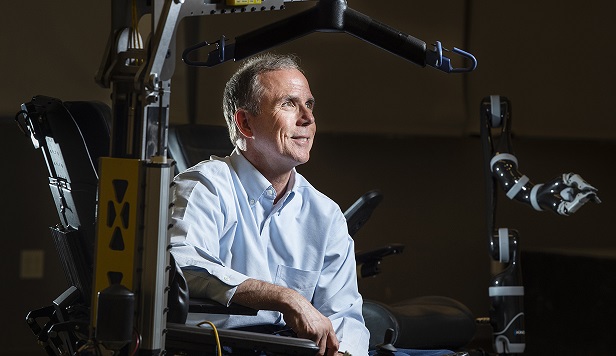
Inventor and assistive technology researcher Rory Cooper, PhD, has been appointed the University of Pittsburgh’s first-ever assistant vice chancellor for research for STEM-health sciences collaborations.
In this role, Dr. Cooper who is an affiliated faculty member of the McGowan Institute for Regenerative Medicine, will provide intellectual leadership to help connect the STEM and health science areas and draw on Pitt’s strengths in these fields. He will also participate in the University Research Council and work to develop institutional-level funding to support research.
“We created this post because Pitt offers an incredible diversity of modes of research and creative endeavors, and corresponding ranges of research and creative products,” said Rob A. Rutenbar, PhD, senior vice chancellor for research at Pitt. “Our office seeks to promote and engage with faculty working across all of our knowledge domains. Creating this position helps us to expand opportunities for research at the intersections of STEM and the health sciences.”
Dr. Rutenbar added that Dr. Cooper is “ideally suited” for this new role.
“His track record in partnerships and interdisciplinary collaborations in these areas is exemplary,” he said.
Dr. Cooper is FISA & Paralyzed Veterans of America Professor and Distinguished Professor of Pitt’s Department of Rehabilitation Science and Technology, as well as professor of bioengineering, physical medicine and rehabilitation, and orthopaedic surgery. Additionally, he is the founding director and VA Senior Research Career Scientist of the Human Engineering Research Laboratories (HERL).
Dr. Cooper has authored or co-authored more than 375 peer-reviewed journal publications, has 30 patents awarded or pending of advanced mobility devices, robotics, and assistive technologies. These include a wheelchair powered by compressed air, a robotic arm extension to help wheelchair users grab items, and a specialized computer mouse for people with prosthetic arms.
He is also an elected fellow of the National Academy of Inventors, the American Association for the Advancement of Science, and several other professional societies.
Dr. Cooper said sees his new appointment as “an opportunity to build new collaborative efforts and to help colleagues conduct more team research of high impact by working collectively.”
“I’m excited to be joining the Pitt Research team and to grow STEM-health sciences collaboration to meet the potential of our faculty, staff and students,” he said.
Illustration: Aimee Obidzinski/University of Pittsburgh.
Dr. Anne Robertson Named Inaugural SSOE Associate Dean of Faculty Development

McGowan Institute for Regenerative Medicine affiliated faculty member Anne Robertson, PhD, Professor in the Department of Mechanical Engineering and Materials Science, will be the inaugural Associate Dean of Faculty Development in the Swanson School of Engineering (SSOE) at the University of Pittsburgh, a role that complements her efforts in the Center for Faculty Excellence, which she established and directs.
The Center aids in the development of junior faculty within the SSOE. This has assisted the promotions of numerous junior faculty members and is a strong selling point for attracting new faculty hires. Her new role allows her to expand her focus to include Associate Professors and Appointment Stream faculty.
The Center has already seen much success via team faculty mentoring, peer-to-peer mentoring, and professional-development workshops. Each junior faculty participating in the mentorship program is assigned a 4-5 senior faculty mentoring team. To date, there have been over 135 mentoring meetings with approximately 90 mentors drawn from across the SSOE, School of Medicine, Dieter School of Arts and Sciences, and a number of departments at Carnegie Mellon University. So far, 19 junior faculty have completed the mentorship program, and all have been awarded tenure. Another 26 faculty are currently in the program. This is 100% participation in a completely voluntary program.
Drs. MaCalus Hogan and James Wang Receive J. Leonard Goldner Award for Best Paper

McGowan Institute for Regenerative Medicine affiliated faculty members MaCalus Hogan, MD, MBA, Vice Chair of Education and Residency Program Director in the Department of Orthopaedic Surgery at the University of Pittsburgh Medical Center and Associate Professor in the Departments of Orthopaedic Surgery and Bioengineering at the University of Pittsburgh, and James Wang, PhD, Albert B. Ferguson, Jr., Chair and Vice Chair of Research for the Department of Orthopaedic Surgery at the University of Pittsburgh School of Medicine, Director of the MechanoBiology Laboratory in the Department of Orthopaedic Surgery, and Professor in the Departments of Bioengineering and Physical Medicine and Rehabilitation, are co-authors of the paper entitled “Effect of metformin on development of tendinopathy due to mechanical overloading in an animal model” which received recognition from the American Orthopaedic Foot & Ankle Society (AOFAS) during its 2020 Annual Meeting.
The other authors of the paper are Jianying Zhang, PhD, Feng Li, MD, PhD, Daibang Nie, PhD, and Kentaro Onishi, DO.
The prestigious J. Leonard Goldner Award is given in recognition of the outstanding basic science paper presented at the AOFAS Annual Meeting. Nominated studies are selected by the Program Committee from blinded abstracts submitted for consideration for the scientific program. Established in 1998 with a gift from J. Leonard Goldner, MD, the award is presented to the senior author and includes a framed certificate and monetary award.
Congratulations, Drs. Hogan and Wang!
Dr. Faina Linkov Named 2020 AAAS Fellow

Duquesne University Department Chair and McGowan Institute for Regenerative Medicine affiliated faculty member Faina Linkov, PhD, MPH, has been named a Fellow of the American Association for the Advancement of Science (AAAS) for her contributions to biobehavioral cancer research, global health work, and improving publishing opportunities for scientists in the developing world.
The AAAS, the world’s largest scientific society, elects fellows each year to recognize their efforts to advance science or its applications. A lifetime honor, fellows are selected by their AAAS peers. Dr. Linkov is believed to be the first female faculty member from Duquesne to receive this recognition, furthering the University’s reputation for expanding student horizons by creating opportunities for women in STEM fields. Dr. Linkov serves as a volunteer mentor for women in STEM programs.
“I’m honored to be selected as an AAAS Fellow at a time when the world needs science more than ever,” said Dr. Linkov, chair for the Department of Health Administration and Public Health at Duquesne’s John G. Rangos, Sr. School of Health Sciences. “Whether it’s developing a vaccine for COVID-19, finding better ways to treat cancer or preventing infectious and chronic diseases, scientists are playing a critical role in improving global health.”
Dr. Linkov’s primary research interest has been gynecologic malignancies, where her work focused on investigating the connection between biological markers and cancer risk reduction. She also worked on health services administration research efforts focusing on benign gynecologic disease.
Her recent research interests included biomedical informatics, where she worked on several cancer registries-based projects with the aim of using existing reportable data to help improve medical efficiency and public health. Her most recent study found that ovarian cancer patients treated with intraperitoneal chemotherapy experienced improved 10-year survival rates.
Dr. Linkov, who joined Duquesne in July after serving as an associate professor at the University of Pittsburgh School of Medicine, also helped to shape global health education as a part of the Global Health Network Supercourse project.
She is a member of the American Association for Cancer Research. In 2012, she received the University of Pittsburgh’s Cancer Institute Scholar Award for meritorious biobehavioral research. She has published over 100 peer reviewed papers and chapters on various aspects of gynecologic disease, epidemiology, education, and public health and is the founding editor-in-chief of the Central Asian Journal of Global Health.
Dr. Linkov received her doctoral degree in epidemiology from the University of Pittsburgh Graduate School of Public Health and completed her post-doctoral training at Pitt in the School of Rehabilitation Science.
Congratulations, Dr. Linkov!
McGowan Institute Faculty Teams Win Pitt Innovation Challenge

The Pitt Innovation Challenge (PInCh®) awarded a total of $485,000 to nine projects that propose creative solutions to address health problems including diagnostics, treatments, and interventions. Four of the winning projects include McGowan Institute for Regenerative Medicine affiliated faculty members on their teams. The challenge, which is in its seventh year, was sponsored by the University of Pittsburgh’s Clinical and Translational Science Institute (CTSI).
Nine project teams progressed to the final round where they pitched their ideas to a panel of judges to win up to $100,000. All finalists were guaranteed a minimum of $25,000 and one year of project management support.
This year, the CTSI incentivized solutions to address aspects of an epidemic or pandemic by offering an additional bonus award up to $25,000. Additional non-pandemic bonus awards were granted based on input from the judges.
The McGowan Institute faculty teams include:
$100,000 winners:
Acoustic Waveform Respiratory Evaluation (AWARE)
A smartphone app that enables at-home lung function monitoring for people with lung disorders such as asthma, COPD, and COVID-19. [Pandemic bonus awardee. Receives additional $25,000 for a total of $125,000.]
- Erick Forno, MD, MPH, Assistant Professor of Pediatrics, University of Pittsburgh School of Medicine and UPMC Children’s Hospital of Pittsburgh
- Wei Chen, PhD, Associate Professor of Pediatrics, Biostatistics, and Human Genetics, University of Pittsburgh
- Wei Gao, PhD, Associate Professor, Electrical and Computer Engineering, University of Pittsburgh Swanson School of Engineering
REPLICA: 3D-Sculpted Cartilage Implants
A custom-made cartilage ear implant that decreases complexity and operative time of facial surgeries, creating a state-of-the-art, high-precision cartilage milling process.
- Jesse Goldstein, MD, Craniofacial Plastic Surgeon, Associate Program Director of the Plastic Surgery Residency at the Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center
- Burak Ozdoganlar, PhD, Ver-Planck Endowed Chair and Professor of Mechanical Engineering, Associate Director of the Engineering Research Accelerator at Carnegie Mellon University
- Liliana Camison, MD, Senior Plastic Surgery Resident, University of Pittsburgh Medical Center
- Lucas Dvoracek, MD, Senior Plastic Surgery Resident, University of Pittsburgh Medical Center
- Phil Campbell, PhD, Professor of Biomedical Engineering and Institute for Complex Engineered Systems, Carnegie Mellon University
- Toygun Cetinkaya, PhD, Student at Carnegie Mellon University
$25,000 winners:
A novel EEG lead clip designed for people with coarse and curly hair, bringing innovative human-centered design to provide a 15x improvement in measurement accuracy for epilepsy, neurological disorder, stroke, and brain injuries in the Black population.
- Christina Patterson, MD, PI, Director of Epilepsy Services and the Epilepsy Monitoring Unit, UPMC Children’s Hospital
- Arnelle Etienne, BS, Co-PI, Director of Accessibility, Precision Neuro; Sevo electrodes lead inventor
- Pulkit Grover, PhD, Co-PI, Associate Professor, Electrical and Computer Engineering, Biomedical Engineering, Neuroscience Institute, Carnegie Mellon University; CTO, Precision Neuro
- Shawn Kelly, PhD, CEO, Precision Neuro; Senior Systems Scientist, Engineering Research Accelerator, Electrical Engineering, Biomedical Engineering, Carnegie Mellon University
- Ashwati Krishnan, PhD, Hardware Lead, Precision Neuro, Carnegie Mellon University
- Amber (Momi) Afelin, BA, Lead Electrode Designer, Precision Neuro, majored in Neuroscience and Behavior, Wesleyan University
- Jasmine Kwasa, PhD student, Electrical & Computer Engineering, Carnegie Mellon University
A software decision support solution that matches specific pediatric organ donor and recipient characteristics in real-time, saving time and reducing the deaths of children waiting for an organ transplant.
- George Mazariegos, MD, Professor of Surgery and Critical Care Medicine, University of Pittsburgh; Chief of Pediatric Transplantation, UPMC Children’s Hospital of Pittsburgh
- Eric Pahl is the founder of OmniLife, a software development and analytics company serving the organ procurement and transplantation industry with mobile and real-time clinical communication and decision support tools
- Jim Squires, MD, Assistant Professor of Pediatrics, University of Pittsburgh
- Kyle Soltys, MD, Associate Professor of Surgery, University of Pittsburgh
- CJ Confair will coordinate Starzl Network project activities specific to Transplant for Kids and coordinate network dissemination
- Alex Kepler is the Research Coordinator at UPMC Children’s Hospital of Pittsburgh and will handle all regulatory documents related to the project
Congratulations, all!
Illustration: University of Pittsburgh’s Clinical and Translational Science Institute.
Two McGowan Institute Faculty Team Projects Continue in the Michael G. Wells Competition

The Michael G. Wells Competition is a part of the Pitt Ventures Student Challenge sponsored by the Michael G. Wells Entrepreneurial Scholars Fund and is for University of Pittsburgh students who are developing innovations related to the healthcare field. In 2020, the competition is celebrating its 10-year anniversary.
Students this year presented their innovations virtually at the Innovation Showcase to compete for the $20,000 Michael G. Wells Entrepreneurial Scholars Award to help move their idea towards commercialization. A $10,000 second place and $5,000 third place prizes are also awarded. The Innovation Showcase is an annual event hosted by the Innovation Institute that provides University innovators the opportunity to present their ideas to an audience of investors, entrepreneurs, regional economic development representatives, local business executives, and Pitt alumni.
This year, after deliberations by the judges, it was determined that there was not a clear winner. However, they felt that with a little more time and money a winner may become certain. These teams received $5000 to continue work and to recompete in the Spring for the remaining prize funds:
Biocarpet
The Biocarpet is a new endovascular device that can be thermoformed to treat peripheral arterial disease occurring in small and complex anatomies, including lesions occurring across joints.
Jonathan Vande Geest, PhD, Professor, Department of Bioengineering
Sneha Jeevan, Translational Lead, Department of Bioengineering
William Wagner, PhD, Director, McGowan Institute for Regenerative Medicine, and Distinguished Professor of Surgery, Bioengineering and Chemical Engineering
John Pacella, MD, Interventional Cardiologist at UPMC and Associate Professor in the School of Medicine
An innovative endovascular device that provides oxygenated blood flow to critical abdominal organs to maintain organ health for transplant harvesting.
Youngjae Chun, PhD, Associate Professor, Departments of Industrial Engineering and Bioengineering
Moataz Elsisy, PhD Student, Industrial Engineering
Bryan Tillman, MD, PhD, Director of Vascular Research, Ohio State College of Medicine.
Congratulations to both teams for the opportunity to move their projects forward.
Happy Holidays from the McGowan Institute

We wish a safe and happy holiday to all!
To the 250+ scientists, engineers and clinicians that are part of the team of professionals who are committed to the development and translation of regenerative medicine and medical device-based therapies;
To the dedicated support staff who make it possible for the scientists, engineers and clinicians to pursue the goals and objectives of the Institute;
To the hundreds of student trainees who are learning the fundamentals and developing the applications that continue to make regenerative medicine the promise of the future in improved health care and quality of life;
To the many agencies, foundations, companies, and individual donors without whose fiscal support and encouragement the outcomes that we give thanks for would not have been possible, and;
To the many patients who have trusted our team. These individuals are the true pioneers and the heart of the achievements we recognize…
Since the formation of the McGowan Center for Artificial Organ Development (1992) and the McGowan Institute for Regenerative Medicine (2001) there have been many accomplishments advancing the science and clinical achievements in regenerative medicine-based therapies. We see progress in the areas of medical devices, tissue engineering and cell-based therapies, and the prospects for the future are many. Thanks for the opportunities and outcomes created by all who participate in or support the initiatives of the McGowan Institute. Best wishes for another successful year!
Regenerative Medicine Podcast Update
The Regenerative Medicine Podcasts remain a popular web destination. Informative and entertaining, these are the most recent interviews:
#216 –– Dr. Peter Alexander discusses his research in the development of in vitro and in vivo models in orthopaedics.
Visit www.regenerativemedicinetoday.com to keep abreast of the new interviews.
PUBLICATION OF THE MONTH
Author: Jeremy J. Velazquez, Ryan LeGraw, Farzaneh Moghadam, Patrick Cahan, Samira Kiani, Mo R. Ebrahimkhani
Title: Gene Regulatory Network Analysis and Engineering Directs Development and Vascularization of Multilineage Human Liver Organoids
Summary: Pluripotent stem cell (PSC)-derived organoids have emerged as novel multicellular models of human tissue development but display immature phenotypes, aberrant tissue fates, and a limited subset of cells. Here, we demonstrate that integrated analysis and engineering of gene regulatory networks (GRNs) in PSC-derived multilineage human liver organoids direct maturation and vascular morphogenesis in vitro. Overexpression of PROX1 and ATF5, combined with targeted CRISPR-based transcriptional activation of endogenous CYP3A4, reprograms tissue GRNs and improves native liver functions, such as FXR signaling, CYP3A4 enzymatic activity, and stromal cell reactivity. The engineered tissues possess superior liver identity when compared with other PSC-derived liver organoids and show the presence of hepatocyte, biliary, endothelial, and stellate-like cell populations in single-cell RNA-seq analysis. Finally, they show hepatic functions when studied in vivo. Collectively, our approach provides an experimental framework to direct organogenesis in vitro by systematically probing molecular pathways and transcriptional networks that promote tissue development.
Source: Cell Systems. 2020 Nov 30;S2405-4712(20)30420-8. Online ahead of print.
GRANT OF THE MONTH
PIs: David Vorp
Title: Preclinical Optimization and Design for Manufacturability of Immunoregulatory Tissue-Engineered Vascular Graft
Description: Despite the emergence of a few companies in recent years based on the clinical translation of small diameter tissue-engineered vascular grafts (TEVGs), the gold standard for arterial bypass in the clinic continues to be autologous vein or artery grafts. Our group has developed, with previous NIH funding, TEVGs based on the documented pro-regenerative immunoregulatory potency of mesenchymal stem/stromal cells (MSCs) and tubular, biodegradable scaffolds. MSCs are immunoregulatory in that they both recruit host neutrophils and macrophages via secreted factors and modulate the recruited cells to a tolerant and pro-regenerative phenotype. We have also demonstrated that MSCs can stimulate the production of new functional vascular extracellular matrix both in-vitro and in-vivo and that MSCs play an acute antithrombogenic role in our TEVGs. Our published work has rigorously tested the ability of human-derived MSCs to induce remodeling of TEVG constructs when implanted as rat aortic interposition grafts. In very recent unpublished work (embargoed pending IP protection), we have also successfully tested in the same rat model cell-free TEVG strategies based on immunoregulatory factors secreted by MSCs. These have included the use of cytokine- and MSC secreted factor-loaded microspheres and MSC-derived extracellular vesicles (EVs) loaded into TEVG scaffolds. We define our TEVG strategies in terms of feasible combinations of “payload” (MSCs, cytokine-loaded microspheres, MSC secreted factor-loaded microspheres, and MSC-derived EVs) loaded into three different types of scaffolds (poly(ester urethane)urea (PEUU), lyophilized silk fibroin (LyoGel), and bilayered porous silk). We have also begun to scale-up the fabrication processes of these TEVG configurations in anticipation of eventual large animal testing and have demonstrated success with a sheep implant pilot study in anticipation of this proposal submission. Our proposed milestone-driven project is ideal for this two-phase Catalyze grant mechanism and successful completion will lead to significant progress toward the clinical translation of our TEVG technology. The R61 phase has two objectives with milestones that will allow us to fully evaluate, in our well-established and relatively high-throughput rat model, all feasible TEVG configurations. The primary outcome of the R61 Phase will be to identify the best combination(s) of immunoregulatory payload and scaffold for a TEVG construct that can most optimally remodel in-vivo into a native-like artery. The R33 phase has five objectives with milestones that will first demonstrate successful fabrication of scaled-up versions of the TEVG configurations that meet the milestones of the R61 phase, and then perform large animal testing of the best (up to four) configuration(s). The outcome of this phase will be to identify the optimal TEVG configuration to move forward toward clinical testing and commercialization, and also to address design for manufacturability and development of regulatory and business plans and connections.
Source: National Heart, Lung, and Blood Institute
Term: August 15, 2020 – July 31, 2022
Amount: $367,238 (one year)
